Abstract
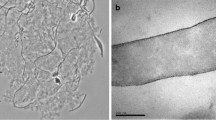
A new anaerobic thermophilic Gram-positive, nonsporeforming bacterium strain ZE-1 was isolated from a hot spring of Kamchatka (USSR). The cells are rod-shaped, (0.5–0.8 · 2.0–20 μm), non-motile. The bacterium can grow between 42 and 75°C; the optimal temperature is 65°C. The growth is possible between pH values 5.0 and 8.5; optimal pH is 7.0. The cultures grow on the media containing peptone, yeast extract, or casein hydrolysate as nitrogen sources in the presence of glucose or some other sugars, mannitol or starch. The main fermentation products of glucose are ethanol, acetate, lactate, H2, CO2; byproducts are propionic, butyric and isovaleric acids. Glucose is metabolized via Embden-Meyerhoff-Parnas pathway. Molecular hydrogen does not inhibit growth. The bacterium does not reduce aceton to isopropanol, but is able to form H2S from elemental sulfur. The bacterium contains a soluble hydrogenase. This enzyme catalyzes both evolution and uptake of H2 and is active in the presence of methyl viologen. The DNA-base composition is 34.6 mol%; the genome size 2.08x109 D. The name proposed for the isolated bacterium strain ZE-1 is Thermoanaerobium lactoethylicum spec. nov.
Similar content being viewed by others
References
Ben-Bassat A, Zeikus JG (1981) Thermobacteroides acetoethylicus gen. nov. and spec. nov., a new chemoorganotrophic, anaerobic, thermophilic bacterium. Arch Microbiol 128:365–370
Bergmeyer HU (1974) Methoden der enzymatischen Analyse. Verlag Chemie, Weinheim
DeLey J, Cattoir H, Reynaerts A (1970) The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem 12:133–142
Fox GE, Stackebrandt E, Hespell RB, Gibson J, Maniloff J, Dyer TA, Wolfe RS, Balch WE, Tanner R, Magrum L, Zablen LB, Blakemore R, Gupta R, Bonen L, Lewis BJ, Stahl DA, Luehrsen KR, Chen KN, Woese CR (1980) The phylogeny of prokaryotes. Science 209:457–463
Gillis M, De Ley J, De Cleene M (1970) The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem 12:143–153
Gogotov IN, Mitkina TV, Glinski VP (1974) Effect of ammonium on hydrogen evolution and nitrogen fixation in Rhodopseudomonas palustris. Microbiologiya 43:586–591 (in Russian)
Gottschalk G (1979) Bacterial metabolism. Springer, Berlin Heidelberg New York
Huber R, Langworthy TA, König H, Thomm M, Woese CR, Sleytr UB, Stetter KO (1986) Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90°C. Arch Microbiol 144:324–333
Krasilnikova EN, Zorin NA, Zacharova EV (1987) The glucose metabolism and the hydrogenase activity of Thermoanaerobium lactoethylicum. Microbiologiya 56:533–536 (in Russian)
Krivenko VV, Chernych NA, Zacharova EV, Duda VI (1987) Some genotypical properties of the anaerobic thermophilic chemoorganotrophic bacteria. Microbiologiya 56:855–860 (in Russian)
Krivenko VV, Bonch-Osmolovskaya EA, Vadachkoriya RM, Miroshnichenko MA (1988) The elemental sulfur reduction by the anaerobic thermophilic chemoorganotrophic bacteria. Microbiologiya 57:660–663 (in Russian)
Lamed R, Zeikus JG (1980) Glucose fermentation pathway of Thermoanaerobium brockii. J Bacteriol. 141:1251–1257
Lamed R, Zeikus JG (1981) Novel NADP-linked alcohol-aldehyde/ketone oxidoreductase in thermophilic ethanologenic bacteria. Biochem J 195:183–190
Leigh JA, Mayer F, Wolfe RS (1981) Acetogenium kivui, gen. nov., spec. nov., a new thermophilic hydrogen-oxidizing, acetogenic bacterium. Arch Microbiol 129:275–280
Leighton L, Himmel M, Burris N, Ng T (1982) Xylan-fermenting anaerobes isolated from acidic, thermal areas. Abst Ann Mtg Amer Soc Microbiol I 18
Lowry CH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J Mol Biol 3:208–218
Marmur J, Doty P (1962) Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol 5:109–118
Morgan HW, Patel BKC, Daniel RM (1985) Comparison of a Thermoanaerobium sp. from a New Zealand hot spring with Thermoanaerobium brockii. FEMS Microbiol Lett 30:121–124
Ng TK, Zeikus JG (1982) Differential metabolism of cellobiose and glucose by Clostridium thermocellum and Clostridium thermohydrosulfuricum. J Bacteriol 150:1391–1399
Patel BKC, Morgan HW, Daniel RM (1985) Fervidobacterium nodosum gen. nov. and spec. nov., a new chemoorganotrophic, caldo-active, anaerobic bacterium. Arch Microbiol 141:63–69
Reynolds ES (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol 17:208–212
Ryter A, Kellenberger E, Birsch-Andersen A, Maaloe Z (1958) Étude en microscope éléctronique de plasmas contenant de l'acide desoxyribonucléique. Z Naturforsch B 136:597–603
Schleifer KH, Stackebrand E (1983) Molecular systematics of prokaryotes. Ann Rev Microbiol 37:143–188
Vadachkoriya RM, Lysenko AM, Krivenko VV (1987) Anaerobic thermophilic chemoorganotrophic bacteria from the hot springs of Kamchatka. Microbiologiya 56:1057–1059 (in Russian)
Weimer PY, Wagner W, Knowlton S, Ng TK (1984) Thermophilic anaerobic bacteria which ferment hemicellulose: characterization of organisms and identification of plasmids. Arch Microbiol 138:31–36
Wiegel JKW, Ljungdahl LG (1981) Thermoanaerobacter ethanolicus gen. nov., spec. nov., a new, extreme thermophilic, anaerobic bacterium. Arch Microbiol 128:343–348
Wiegel JWK (1986) Genus Thermoanaerobacter. In: Holt JG (ed) Bergey's manual of systematic bacteriology, vol 2, 9th edn. Williams & Williams, Baltimore, pp 1379–1383
Woese CR (1987) Bacterial evolution. Microbiol Rev 51:221–271
Zacharova EV, Krivenko VV, Mitjuschina LL, Duda VI (1987) A new obligately thermophilic anaerobic bacterium. Microbiologiya 56:288–293 (in Russian)
Zeikus JG, Hegge PW, Anderson MA (1979) Thermoanaerobium brockii gen. nov. and sp. nov., a new chemoorganotrophic, caldoactive, anaerobic bacterium. Arch Microbiol 122:41–48
Zhilina TN, Zavarzin GA (1978) The cultivation methods of methanogenic bacteria. In: Theoretical and methodical basis of anaerobic microorganisms investigation. Pushchino, pp 68–88 (in Russian)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kondratieva, E.N., Zacharova, E.V., Duda, V.I. et al. Thermoanaerobium lactoethylicum spec. nov. a new anaerobic bacterium from a hot spring of Kamchatka. Arch. Microbiol. 151, 117–122 (1989). https://doi.org/10.1007/BF00414424
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00414424