Abstract
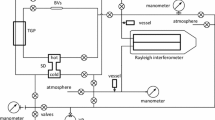
The concentration diffusion coefficient, D 12, is measured for the equimolar mixtures of Ne-Ar, Ne-Xe, Ne-H2, Xe-H2, H2-N2 and H2-O2 binary gas systems in a two-bulb metal apparatus in the temperature range 0 C to 100 C. These values are compared with the existing data on these systems and with the predictions of the kinetic theory in conjunction with the modified Buckingham exp-six potential. Unlike the thermal diffusion coefficient, with the simple theory it is possible to predict D 12 within a few percent even for systems involving polyatomic gases. The smoothed experimental D 12 values are also used to obtain data for the coefficients of viscosity and thermal conductivity at round temperatures and compositions for these systems.
Similar content being viewed by others
Abbreviations
- C t2 :
-
relative amount of a gas in the mixture in the bulb 2 at an instant t
- C ∞2 :
-
relative amount of the same gas in the mixture in the bulb 2 at equilibrium
- D 12 :
-
diffusion coefficient
- X 1 :
-
mole-fraction of the heavier component in the mixture
- η mix :
-
viscosity coefficient
- λ mix :
-
thermal conductivity coefficient
References
Weissman, S., S. C. Saxena, and E. A. Mason, Phys. Fluids 3 (1960) 510.
Weissman, S., S. C. Saxena, and E. A. Mason, Phys. Fluids 4 (1961) 643.
Saxena, S. C., M. P. Saksena, and R. S. Gambhir, Brit. J. Appl. Phys. 15 (1964) 843.
Saxena, S. C., M. P. Saksena, R. S. Gambhir, and J. M. Gandhi, Physica 31 (1965) 333.
Mathur, B. P. and S. C. Saxena, Z. Naturforschg. 22a (1967) 164.
Nain, V. P. S. and S. C. Saxena, J. Chem. Phys. 51 (1969) 1541.
Mathur, B. P. and S. C. Saxena, Appl. Sci. Res. 18 (1968) 325.
Saxena, V. K., V. P. S. Nain, and S. C. Saxena, J. Chem. Phys. 48 (1968) 3681.
Chapman, S. and T. G. Cowling, The Mathematical Theory of Non-Uniform Gases, Cambridge University Press, Reprinted Second Edition, 1953.
Mason, E. A., J. Chem. Phys. 22 (1954) 169.
Srivastava, B. N. and K. P. Srivastava, J. Chem. Phys. 30 (1959) 984.
Srivastava, K. P. and A. K. Barua, Indian J. Phys. 33 (1959) 229.
Schafer, K. and K. Schumann, Z. Electrochem. Ber. Bunsenges. Physik. Chem. 61 (1957) 246.
Paul, R. and I. B. Srivastava, J. Chem. Phys. 35 (1961) 1621.
Schäfer, K., H. Corte, and H. Moesta, Z. Electrochem. Angew. Physik. Chem. 55 (1951) 662.
Boardman, L. E. and N. E. Wild, Proc. Roy. Soc. London A 162 (1937) 511.
Scott, D. S. and K. E. Cox, Can. J. Chem. Eng. 38 (1960) 201.
Giddings, J. C. and S. L. Seager, Ind. Eng. Chem. Fundamentals 1 (1962) 277.
Waldman, L., Naturwissenschaften 32 (1944) 223.
Van Heijninge, R. J. J., A. Feberwee, A. van Oosten, and J. J. M. Beenakker, Physica 32 (1966) 1649.
Walker, R. S. and A. A. Westenberg, J. Chem. Phys. 32 (1960) 436.
Hirschfelder, J. O., Heat Conductivity in Polyatomic, Electronically Excited, or Chemically Reacting Mixtures III, Sixth International Symposium on Combustion, New York: Reinhold, (1957) 351.
Gandhi, J. M. and S. C. Saxena, Proc. Phys. Soc. 87 (1966) 273.
Mathur, S. and S. C. Saxena, Proc. Phys. Soc. 89 (1966) 753.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nain, V.P.S., Saxena, S.C. Measurement of the concentration diffusion coefficient for Ne-Ar, Ne-Xe, Ne-H2, Xe-H2, H2-N2 and H2-O2 gas systems. Appl. Sci. Res. 23, 121–133 (1971). https://doi.org/10.1007/BF00413191
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00413191