Summary
-
1.
The occurrence of a l-valine carboxy-lyase in Bacillus sphaericus has been demonstrated. The enzyme was found to occur in four out of nine strains tested.
-
2.
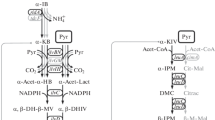
Some properties of the decarboxylase were studied in detail using acetonedried cells of B. sphaericus ATCC 245. The substrate-unspecific enzyme decarboxylates the following amino acids: valine, norvaline, leucine, 2-amino n-butyric acid, norleucine, isoleucine, methionine, alanine and phenylalanine. When two substrates are present simultaneously no additive effects are detected.
-
3.
The pH optimum for the reactions is about pH 7.7–7.9, the optimum temperature between 50 and 60°C.
-
4.
The enzyme is activated by pyridoxal phosphate (PLP) and, to a lower extent, by pyridoxal. In the absence of added PLP the rate of decarboxylation decreases continuously. The inactivation occurs more rapidly during decarboxylation of leucine than of valine; added leucine progressively inhibits valine decarboxylation.
-
5.
The decaroxylations are sensitive to reagents known to combine with carbonyl and — SH groups. PLP protects the enzyme to some extent against a progressive inhibition by iodoacetate which is stronger with leucine than with valine. It doesn't, however, reverse inactivation already established.
-
6.
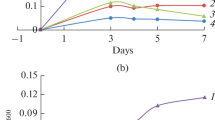
The enzyme activity is highest in very young cultures of Bacillus sphaericus. It decreases rapidly in the early exponential phase of growth.
Zusammenfassung
-
1.
In 4 von 9 untersuchten Stämmen von Bacillus sphaericus ließ sich eine l-Valin-Carboxylyase nachweisen.
-
2.
Einige Eigenschaften des Enzyms wurden an Acetonpräparaten von B. sphaericus ATCC 245 näher untersucht. Die Decarboxylase reagiert substratunspezifisch mit folgenden Aminosäuren: Valin, Norvalin, Leucin, 2-Amino-n-buttersäure, Norleucin, Isoleucin, Methionin, Alanin und Phenylalanin. Bei gleichzeitigem Angebot zweier Substrate läßt sich kein additiver Effekt feststellen.
-
3.
Das pH-Optimum der Enzymkatalyse liegt bei pH 7,7–7,9, das Temperaturoptimum zwischen 50 und 60°C.
-
4.
Die Decarboxylase wird durch Pyridoxalphosphat (PLP), in geringerem Maße auch durch Pyridoxal, aktiviert. Ohne Zusatz von PLP nimmt die Decarboxylierungsrate stetig ab, und zwar bei Leucin sehr viel schneller als bei Valin; die Decarboxylierung von Valin wird durch Leucin gehemmt.
-
5.
Carbonyl- und SH-Gruppenreagentien hemmen die Decarboxylase. PLP schützt das Enzym in gewissem Umfang gegen die bei Leucin stärker als bei Valin progressiv zunehmende Hemmung durch Jodacetat, kann aber eine bereits eingetretene Inaktivierung nicht wieder aufheben.
-
6.
Die Enzymaktivität ist am höchsten in ganz jungen Kulturen von Bacillus sphaericus; schon zu Beginn des exponentiellen Wachstums fällt sie steib ab.
Similar content being viewed by others
Literatur
Antia, M., Work, E.: Oxidation of meso-α,ɛ-diaminopimelic acid by certain sporulating species of bacteria. J. gen. Microbiol. 26, 67–80 (1961).
Bast, E.: Über Vorkommen und Entstehung flüchtiger primärer Amine bei Bakterien. Arch. Mikrobiol. 79, 7–11 (1971).
Crocomo, O. J., Fowden, L.: Amino acid decarboxylases of higher plants: the formation of ethylamine. Phytochemistry 9, 537–540 (1970).
Ekladius, L., King, H. K., Sutton, C. R.: Decarboxylation of neutral amino acids in Proteus vulgaris. J. gen. Microbiol. 17, 602–619 (1957).
Grandgenett, D. P., Stahly, D. P.: Diaminopimelate decarboxylase of sporulating bacteria. J. Bacteriol. 96, 2099–2109 (1968).
Hagino, H., Nakayama, K.: Amino acid metabolism in microorganisms. II. Production of 3-methylthiopropylamine from methionine by certain strains of Streptomyces. Agr. biol. Chem. (Tokyo) 31, 1367–1371 (1967).
——: Amino acid metabolism in microorganisms. IV. l-Methionine decarboxylase produced by a Streptomyces strain. Agr. biol. Chem. (Tokyo) 32, 727–733 (1968).
Hartmann, T.: Zur Biogenese flüchtiger Amine beim Mutterkorn-Pilz Claviceps purpurea. Planta (Berl.) 66, 191–206 (1965).
Hartmann, T.: Leucin-Carboxylyase aus marinen Rhodophyceae: Vorkommen, Verbreitung und einige Eigenschaften des Enzyms. Phytochemistry (im Druck, 1971 a).
Hartmann, T.: Leucin-Carboxylyase aus marinen Rhodophyceae: 2. Eigenschaften des Enzyms aus Polysiphonia urceolata. Biochem. Physiol. Pflanzen (im Druck, 1971 b).
Hartmann, T.: Leucin-Carboxylyase aus marinen Rhodophyceae: 3. Coenzymspezifität und Aktivierung durch Carbonylverbindungen. Biochem. Physiol. Pflanzen (im Druck, 1971 c).
— Bast, E.: Decarboxylierung von l-Methionin durch Proteus vulgaris und Bacillus sphaericus. Arch. Mikrobiol. 64, 239–243 (1969).
King, H. K.: Studies on leucine decarboxylase. In: Chemical and biological aspects of pyridoxal catalysis. (Ed. by E. E. Snell, P. M. Fasella, A. Braunstein and A. Rossi Fanelli.) I. U. B. Symp. Ser. Vol. 30, pp. 253–266. Oxford-London-New York-Paris: Pergamon Press 1963.
Lovenberg, W., Weissbach, H., Udenfriend, S.: Aromatic l-amino acid decarboxylase. J. biol. Chem. 237, 89–93 (1962).
McGilvery, R. W., Cohen, P. P.: The decarboxylation of l-phenylalanine by Streptococcus faecalis R. J. biol. Chem. 174, 813–816 (1948).
Meadow, P., Work, E.: The effects of vitamin B6 and its derivatives on diaminopimelic acid decarboxylase in Bacillus sphaericus asporogenous. Biochim. biophys. Acta (Amst.) 29, 180–187 (1958).
Patte, J.-C., Loviny, T., Cohen, G. N.: Répression de la décarboxylase de l'acide méso-α,ɛ-diaminopimélique par la l-lysine, chez Escherichia coli. Biochim. biophys. Acta (Amst.) 58, 359–360 (1962).
Powell, J. F.: The changes in the total vitamin B6 and the pyridoxal phosphate content of cells of Bacillus sphaericus during growth and sporulation: their possible relationships with α,ɛ-diaminopimelic acid metabolism. Biochem. J. 70, 91–96 (1958).
— Strange, R. E.: α,ɛ-Diaminopimelic acid metabolism and sporulation in Bacillus sphaericus. Biochem. J. 65, 700–708 (1957).
Richardson, M.: Studies on the biogenesis of some simple amines and quaternary ammonium compounds in higher plants. Isoamylamine and isobutylamine. Phytochemistry 5, 23–30 (1966).
Schales, O., Schales, S. S.: Glutamic acid decarboxylase of higher plants. II. pH-activity curve, reaction kinetics, inhibition by hydroxylamine. Arch. Biochem. 11, 155–166 (1946).
Simon, E. W.: Valine decarboxylation in Arum spadix. J. exp. Bot. 13, 1–4 (1962).
Steiner, M., Hartmann, T.: Über Vorkommen und Verbreitung flüchtiger Amine bei Meeresalgen. Planta (Berl.) 79, 113–121 (1968).
Sutton, C. R., King, H. K.: Inhibition of leucine decarboxylase by thiol-binding reagents. Arch. Biochem. Biophys. 96, 360–370 (1962).
White, P. J., Kelly, B., Suffling, A., Work, E.: Variation of activity of bacterial diaminopimelate decarboxylase under different conditions of growth. Biochem. J. 91, 600–610 (1964).
Author information
Authors and Affiliations
Additional information
Teil einer Dissertation (E. Bast) der Math.-Naturw. Fakultät der Universität Bonn (D 5).
Rights and permissions
About this article
Cite this article
Bast, E., Hartmann, T. & Steiner, M. Über eine Valin-Carboxylyase aus Bacillus sphaericus . Archiv. Mikrobiol. 79, 12–24 (1971). https://doi.org/10.1007/BF00412037
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00412037