Summary
A comparison between the effects of diethylether and urea on the electron transport system of R. rubrum was made.
-
1.
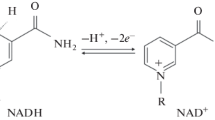
Both reagents cause an increasing and complete inactivation of NADH oxidation with oxygen and cytochrome c as electron acceptors. The flavoprotein NADH dehydrogenase is only slightly affected. The oxidation of succinate (cytochrome c as electron acceptor) is inactivated only by much higher concentrations of either agent, succinate dehydrogenase being completely unaffected. Low concentrations of urea, in contrast to diethylether, stimulate succinate-cytochrome creductase. A preferential attack of both agents on the NADH → ubiquinone segment is suggested.
-
2.
By treatment of the membranes with diethylether NADH dehydrogenase is transformed to a second, less active state. This form of the enzyme is characterized by a slower V max, a slightly increased k m(NADH), and an unchanged k m(electron acceptor). It cannot be reactivated.
-
3.
Urea, like phosphate, causes a conformational change at the active site of succinate dehydrogenase as evidenced from an identical activation behaviour.
-
4.
About 20% of the particulate activity of NADH dehydrogenase can be released from the membranes by treatment with urea. This activity apparently is identical with that solubilized by deoxycholate.
Similar content being viewed by others
References
Boll, M.: Oxydation von reduziertem Nicotinamid-Adenin-dinucleotid in Rhodospirillum rubrum. I. Charakterisierung einer löslichen NADH-dehydrogenase. Arch. Mikrobiol. 62, 94–110 (1968a).
—: Enzyme der Elektronentransportpartikel aus Rhodospirillum rubrum: Eigenschaften von NADH-und Succinat-Cytochrome c-Reductase. Arch. Mikrobiol. 64, 85–102 (1968b).
—: The effect of deoxycholate on enzymes with electron transport function from Rhodospirillum rubrum. Arch. Mikrobiol. 68, 191–200 (1969a).
—: Oxidation of reduced nicotinamide-adenine-dinucleotide in Rhodospirillum rubrum. III. Properties of a NADH dehydrogenase solubilized from electron transport particles. Arch. Mikrobiol. 69, 301–313 (1969b).
—: Studies with Triton X-100 treated electron transport particles from Rhodospirillum rubrum. Arch. Mikrobiol. 71, 1–8 (1970a).
—: Action of sodium dodecylsulfate on electron transport enzymes of Rhodospirillum rubrum. Experientia (Basel) 26, 956–957 (1970b).
—: The effect of proteolytic and lipolytic enzymes on the electron transport particle fraction of Rhodospirillum rubrum. I. Proteases. Z. Naturforsch. 25b, 1448–1450 (1970c).
Boll, M.: Effect of proteolytic and lipolytic enzymes on the electron transport particle fraction of Rhodospirillum rubrum. II. Phospholipases. Z. Naturforsch. (in press) (1971).
Crane, F. L., Widmer, C., Lester, R. L., Hatefi, Y.: Studies on the electron transport system. XV. Coenzyme Q and the succinoxidase activity of the electron transport particle. Biochim. biophys. Acta (Amst.) 31, 476–489 (1959).
Davis, K. A., Hatefi, Y.: Kinetics of the resolution of complex I (NADH-coenzyme Q-reductase) of the mitochondrial electron transport system by chaotropic agents. Biochemistry 8, 3355–3361 (1969).
Eisenberg, R. C., Yu, L., Wolin, M. J.: Divalent cation activation of deoxycholatesolubilized and-inactivated membrane NADH oxidase of Bacillus megaterium KM. J. Bact. 102, 172–177 (1970).
Green, D. E., Tzagoloff, A.: The mitochondrial electron transfer chain. Arch. Biochem. 116, 293–304 (1966).
Hatefi, Y., Haavik, A. G., Griffiths, D. E.: Studies on the electron transfer chain. XL. Preparation and properties of mitochondrial coenzyme Q-reductase. J. biol. Chem. 237, 1676–1680 (1962a).
——, Fowler, L. R., Griffiths, D. E.: Studies on the electron transfer system. XLII. Reconstitution of the electron transfer system. J. biol. Chem. 237, 2661–2669 (1962b).
—, Stempel, K. E.: Resolution of complex I (DPNH-coenzyme Q-reductase) of the mitochondrial electron transfer system. Biochem. biophys. Res. Commun. 26, 301–308 (1967).
Horgan, D. J., Singer, T. P., Casida, J. E.: Studies on the respiratory chainlinked NADH dehydrogenase. XII. Binding sites of rotenone, piericidin A and amytal in the respiratory chain. J. biol. Chem. 243, 834–843 (1968).
Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J.: Protein measurement with the Folin phenol reagent. J. biol. Chem. 193, 265–275 (1951).
Razin, S., Ne'eman, Z., Ohad, I.: Selective reaggregation of solubilized Mycoplasma membrane proteins and the kinetics of membrane reformation. Biochim. biophys. Acta (Amst.) 193, 277–293 (1969).
Takemori, A. L., King, T. E.: Reconstitution of the respiratory chain enzyme system. XIII. Sequential fragmentation of succinate oxidase. Preparation and properties of succinate-cytochrome c-reductase and the cytochrome b-c1 particle. J. biol. Chem. 239, 3546–3558 (1964).
Yu, L., Wolin, M. J.: Factors affecting deoxycholate inactivation and Mg++ reactivation of Bacillus megaterium KM membrane NADH oxidase. J. Bact. 103, 467–474 (1970).
Ziegler, D. M., Doeg, K. A.: Studies on the electron transport system XLIII. The isolation of a succinic coenzyme Q reductase from beef heart mitochondria. Arch. Biochem. 97, 41–50 (1962).
—, Rieske, J. S.: Preparation and properties of succinate-coenzyme Q-reductase (complex II). In: Methods in Enzymology, vol. X, pp. 231–235. R. W. Estabrook and M. Pullman, eds. New York: Academic Press 1967.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boll, M. Structure and function of electron transport systems. Archiv. Mikrobiol. 76, 174–182 (1971). https://doi.org/10.1007/BF00411791
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00411791