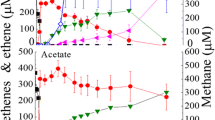
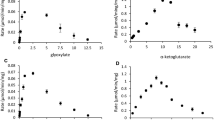
Abstract
The conversion of methanol by cell-free extracts of the acetogenic bacterium Eubacterium limosum was studied. Incubation of mixed cell-free extracts of both E. limosum and Methanobacterium formicicum resulted in methane formation from methanol in the presence of ATP and 2-mercaptoethanesulfonic acid. The separate extracts were not able to perform this reaction. Addition of ferredoxin obtained from Methanosarcina barkeri to the mixed extracts resulted in increased methane formation. The enzyme, responsible for methanol binding in cell-free extract of E. limosum, was inactivated by FAD under N2 and exhibited maximal activity under an atmosphere of H2. This enzyme contains a firmly bound cobalamin which was methylated by methanol in the presence of ATP. It was demethylated in the presence of methylcobalamin: coenzyme M methyltransferase obtained from M. barkeri under concomitant formation of methylated coenzyme M. These properties are similar to those of methanol: 5-hydroxybenzimidazolylcobamide methyltransferase from M. barkeri. It was proposed that methylotrophic acetogens and methylotrophic methanogens use similar enzymes in the first step of methanol conversion.
Similar content being viewed by others
Abbreviations
- HS-CoM:
-
2-mercaptoethanesulfonic acid
- CH3S-CoM:
-
2-(methylthio)ethanesulfonic acid
- BrES:
-
2-bromoethanesulfonic acid
- TES:
-
N-tris(hydroxymethyl)-methyl-2-aminoethanesulfonic acid
- MT1 :
-
methanol: 5-hydroxybenzimidazolylcobamide methyltransferase
- MT2 :
-
methylcobalamin
- HS-CoM:
-
methyltransferase
- DMBI:
-
5,6-dimethylbenzimidazole and HBI, 5-hydroxybenzimidazole, are α-ligands of corrinoids
- (S-CoM)2 :
-
2,2′-dithiodiethanesulfonic acid
References
Bache R, Pfennig N (1981) Selective isolation of Acetobacterium woodii on methoxylated aromatic acids and determination of growth yield. Arch Microbiol 130:255–261
Ellefson WL, Wolfe RS (1980) Role of component C in the methylreductase system of Methanobacterium. J Biol Chem 235:8388–8389
Genthner BRS, Davis CL, Bryant MP (1981) Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol-and H2−CO2-utilizing species. Appl Environm Microbiol 42:12–19
Gunsalus RP, Wolfe RS (1978) ATP activation and properties of the methyl coenzyme M reductase system in Methanobacterium thermoautotrophicum. J Bacteriol 135:851–857
Hermans JMH, Hutten TJ, Drift C van der, Vogels GD (1980) Analysis of coenzyme M (2-mercaptoethanesulfonic acid) derivatives by isotachophoresis. Anal Biochem 106:363–366
Hutten TJ, Jong MH de, Peeters BPH, Drift C van der, Vogels GD (1981) Coenzyme M derivatives and their effects on methane formation from carbon dioxide and methanol by cell extracts of Methanosarcina barkeri. J Bacteriol 145:27–34
Keltjens JT, Whitman WB, Caerteling CG, Kooten AM van, Wolfe RS, Vogels GD (1982) Presence of coenzyme M derivatives in the prosthetic group (coenzyme MF430) of methylcoenzyme M reductase from Methanobacterium thermoautotrophicum. Biochem Biophys Res Commun 108:495–503
Kerby R, Niemczura W, Zeikus JG (1983) Single-carbon catabolism in acetogens: analysis of carbon flow in Acetobacterium woodii and Butyribacterium methylotrophicum by fermentation and 13C nuclear magnetic resonance measurement. J Bacteriol 155: 1208–1218
Lynd LH, Zeikus JG (1983) Metabolism of H2−CO2, methanol, and glucose by Butyribacterium methylotrophicum. J Bacteriol 153:1415–1423
Moore WEC, Cato EP (1965) Synonomy of Eubacterium limosum and Butyribacterium rettgeri: Butyribacterium limosum comb. nov. Int Bull Bact Nomenclat Tax 15:69–80
Müller E, Fahlbusch K, Walther R, Gottschalk G (1981) Formation of N,N-dimethylglycine, acetic acid, and butyric acid from betaine by Eubacterium limosum. Appl Environm Microbiol 42:439–445
Schoberth S (1977) Acetic acid from H2 and CO2. Formation of acetate by cell extracts of Acetobacterium woodii. Arch Microbiol 114:143–148
Sedmak JJ, Grossberg SE (1977) A rapid, sensitive, and versatile assay for protein using coomassie brilliant blue G250. Anal Biochem 79:544–552
Tanner RS, Stackebrandt E, Fox GE, Woese CR (1981) A phylogenetic analysis of Acetobacterium woodii, Clostridium barkeri, Clostridium butyricum, Clostridium lituseburense, Eubacterium limosum, and Eubacterium tenue. Curr Microbiol 5:35–38
Taylor CD, Wolfe RS (1974) A simplified assay for coenzyme M (HSCH2CH2SO3). Resolution of methylcobalamin — coenzyme M methyltransferase and use of sodium borohydride. J Biol Chem 249:4886–4890
Van Bruggen JJA, Zwart KB, Assema RM van, Stumm CK, Vogels GD (1984) Methanobacterium formicicum, an endosymbiont of the anaerobic ciliate Methopus striatus McMurrich. Arch Microbiol (in press)
Van der Meijden P, Heythuysen HJ, Sliepenbeek H, Houwen FP, Drift C van der, Vogels GD (1983a) Activation and inactivation of methanol: 2-mercaptoethane-sulfonic acid methyltransferase from Methanosarcina barkeri. J Bacteriol 153:6–11
Van der Meijden P, Heythuysen HJ, Pouwels A, Houwen FP, drift C van der, Vogels GD (1983b) Methyltransferases involved in methanol conversion by Methanosarcina barkeri. Arch Microbiol 134:238–242
Van der Meijden P, Jansen LP, Drift C van der, Vogels GD (1983c) Involvement of corrinoids in the methylation of coenzyme M (2-mercaptoethanesulfonic acid) by methanol and enzymes from Methanosarcina barkeri. FEMS Microbiol Lett 19:247–251
Van der Meijden P, Lest C van der, Drift C van der, Vogels GD (1984) Reductive activation of methanol: 5-hydroxybenzimidazolylcobamide methyltransferase of Methanosarcina barkeri. Biochem Biophys Res Commun 118:760–766
Welty FK, Wood HG (1978) Purification of the “corrinoid” enzyme involved in the synthesis of acetate by Clostridium thermoaceticum. J Biol Chem 253:5832–5838
Wiegel J, Braun M, Gottschalk G (1981) Clostridium thermoautotrophicum species novum, a thermophile producing acetate from molecular hydrogen and carbon dioxide. Curr Microbiol 5:255–260
Wood HG, Drake HL, Hu SI (1982) Studies with Clostridium thermoaceticum and the resolution of the pathway used by acetogenic bacteria that grow on carbon monoxide or carbon dioxide and hydrogen. Proc Biochem Symp, 29–56
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van der Meijden, P., van der Drift, C. & Vogels, G.D. Methanol conversion in Eubacterium limosum . Arch. Microbiol. 138, 360–364 (1984). https://doi.org/10.1007/BF00410904
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00410904