Abstract
The purpose of this investigation was to characterize the carbohydrate catabolism and the constellation of the respiratory chain components of Haemophilus influenzae RAMC 18 Bensted, H. parainfluenzae 1 Fleming, H. parainfluenzae 429 Pittman and H. aegyptius 180a Pittman. These strains represent several physiological types with respect to respiratory quinones and glucose catabolism.
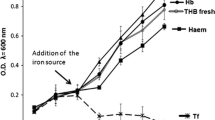
On addition of glucose or lactate to the complex growth medium a remarkable increase in cell mass was observed. Depending on the growth rate, carbohydrate degradation varied with the strains examined so that at the end of the exponential growth phase only small amounts of the supplements could be demonstrated.
All strains were found to possess functional enzymes of Embden-Meyerhof-Parnas-, Entner-Doudoroff-pathways, hexosemonophosphate shunt, tricarboxylic acid cycle and gluconeogenesis with an extremely high activity of malate dehydrogenase.
The concentration of cytochromes varied according to culture conditions. The cytochromes a1, d, o and b+c were found to occur under aerobic conditions. In cells grown anaerobically in the presence of fumarate cytochromes a1 and d could not be demonstrated. Under aerobic conditions preparations of H. parainfluenzae 1 Fleming exhibited an α-maximum at 558 nm, whereas under anaerobic culture conditions with fumarate as terminal electron acceptor an α-maximum at 552 nm occurred, suggesting different roles of b and c type cytochromes in aerobic and anaerobic electron transport to fumarate, respectively.
Similar content being viewed by others
References
Amarasingham, C. R. and Davis, B. D. 1965. Regulation of α-ketoglutarate dehydrogenase formation in Escherichia coli. — J. Biol. Chem. 240: 3664–3668.
Anderson, R. L. and Wood, W. A. 1969. Carbohydrate metabolism in microorganisms. —Annu. Rev. Microbiol. 23: 539–578.
Atkinson, D. E. 1969. Regulation of enzyme function. — Annu. Rev. Microbiol. 23: 47–68.
Bergmeyer, H. U. und Bernt, E. 1974a. Malat-Dehydrogenase, p. 649–653. In H. U. Bergmeyer, (ed.), Methoden der enzymatischen Analyse, 3. Aufl. — Verlag Chemie, Weinheim/Bergstr.
Bergmeyer, H. U. und Bernt, E. 1974b. Lactat-Dehydrogenase, p. 607–612. In H. U. Bergmeyer, (ed.), Methoden der enzymatischen Analyse, 3. Aufl. — Verlag Chemie, Weinheim/Bergstr.
Bücher, T. and Pfleiderer, G. 1955. Pyruvate kinase from muscle, p. 435–440. In S. P. Colowick and N. O. Kaplan, (eds.), Methods in Enzymology, Vol. 1. — Academic Press, New York-London.
Cho, H. W. and Eagon, R. G. 1967. Factors affecting the pathways of glucose catabolism and tricarboxylic acid cycle in Pseudomonas natriegens. — J. Bacteriol. 93: 866–873.
Collins, F. M. and Lascelles, J. 1962. The effect of growth conditions on oxidative and dehydrogenase activity in Staphylococcus aureus. — J. Gen. Microbiol. 29: 531–535.
Englesberg, E., Gibor, A. and Levy, J. B. 1954. Adaptive control of terminal respiration in Pasteurella pestis. — J. Bacteriol. 68: 146–151.
Fraenkel, D. G. and Vinopal, R. T. 1973. Carbohydrate metabolism in bacteria. — Annu. Rev. Microbiol. 27: 69–100.
Gray, C. T., Wimpenny, J. W. T., Hughes, D. E. and Mossman, M. R. 1966a. Regulation of metabolism in facultative bacteria. I. Structural and functional changes in Escherichia coli associated with shifts between the aerobic and anaerobic states. — Biochim. Biophys. Acta 117: 22–32.
Gray, C. T., Wimpenny, J. W. T. and Mossman, M. R. 1966b. Regulation of metabolism in facultative bacteria. II. Effects of aerobiosis, anaerobiosis and nutrition on the formation of Krebs cycle enzymes in Escherichia coli. — Biochim. Biophys. Acta 117: 33–41.
Hanson, R. S. and Cox, D. P. 1967. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. — J Bacteriol. 93: 1777–1787.
Hatchikian, E. C. and Le Gall, J. 1972. Evidence for the presence of a b-type cytochrome in the sulfate-reducing bacterium Desulfovibrio gigas, and its role in the reduction of fumarate by molecular hydrogen. — Biochim. Biophys. Acta 267: 479–484.
Holländer, R. 1976. Physiologie und Physiotaxonomie einiger Vertreter der Gattung Haemophilus Winslow et al. — Ph. D. Thesis, Marburg.
Holländer, R. and Mannheim, W. 1975. Characterization of haemophilic and related bacteria by their respiratory quinones and cytochromes. — Int. J. Syst. Bacteriol. 25: 102–107.
Hugh, R. and Leifson, E. 1953. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. — J. Bacteriol. 66: 24–26.
Hughes, D. E. and Wimpenny, J. W. T. 1969. Oxygen metabolism by micro-organisms. —Adv. Microbiol. Physiol. 3: 197–232.
Kersters, K. and De Ley, J. 1968. The occurrence of Entner-Doudoroff-pathway in bacteria. — Antonie van Leeuwenhoek 34: 393–408.
King, J. 1974. 6-Phosphogluconat Dehydrogenase, p. 668–672. In H. U. Bergmeyer, (ed.), Methoden der enzymatischen Analyse, 3. Aufl. — Verlag Chemie, Weinheim/Bergstr.
King, M. T. and Drews, G. 1973. The function and localization of ubiquinone in the NADH and succinate oxidase system of Rhodopseudomonas palustris. — Biochim. Biophys. Acta 305: 230–248.
Krampitz, L. O. 1961. Cyclic mechanism of terminal oxidations, p. 209–256. In I. C. Gunsalus and R. Y. Stanier, (eds.), The Bacteria, Vol. II. — Academic Press, New York-London.
Kröger, A. 1974. Electron-transport phosphorylation coupled to fumarate reduction in anaerobically grown Proteus rettgeri. — Biochim. Biophys. Acta 347: 273–289.
Kröger, A. and Dadák, V. 1969. On the role of quinones in bacterial electron transport. The respiratory system of Bacillus megaterium. — Europ. J. Biochem. 11: 328–340.
Kröger, A., Dadák, V., Klingenberg, M. and Diemer, F. 1971. On the role of quinones in bacterial electron transport. Differential roles of ubiquinone and menaquinone in Proteus rettgeri. — Europ. J. Biochem. 21: 322–333.
Massey, V. 1955. Fumarase, p. 729–735. In S. P. Colowick and N. O. Kaplan, (eds.), Methods in Enzymology, Vol. I. — Academic Press, New York-London.
Ornston, L. N. 1971. Regulation of catabolic pathways in Pseudomonas. — Bacteriol. Rev. 35: 87–116.
Scrutton, M. C. 1971. Assay of enzymes of CO2 metabolism, p. 479–541. In J. R. Norris and D. W. Ribbons, (eds.), Methods in Microbiology, Vol. 6A. — Academic Press, New York-London.
Strasters, K. C. and Winkler, K. C. 1960. Carbohydrate metabolism in Staphylococcus aureus. — J. Gen. Microbiol. 33: 213–229.
Szarkowska, L. and Klingenberg, M. 1963. On the role of ubiquinone in mitochondria. Spectrophotometric and chemical measurement of its redox reactions. — Biochem. Z. 338: 674–697.
Szulmajster, J. and Hanson, R. S. 1965. Physiological control of sporulation in Bacillus subtilis, p. 162–173. In L. L. Campbell and H. O. Halvorson, (eds.), Spores III. — American Society for Microbiology, Ann Arbor.
Takeo, K. 1969. Existence and properties of two malic enzymes in Eschrichia coli-Especially of NAD-linked enzyme. — J. Biochem. (Tokyo) 66: 379–387.
Umbreit, W. W., Burris, R. H. and Stauffer, J. F. 1964. Manometric techniques, 4th ed. —Burgess Publ. Co., Minneapolis.
Vogel, H. J. 1961. Control by repression, p. 23–65. In D. M. Bonner, (ed.), Control mechanisms in cellular processes. — Ronald Press Co., New York.
de Vries, W., van Wyck-Kapteyn, W. M. C. and Oosterhuis, S. K. H. 1974. The presence and function of cytochromes in Selenomonas ruminantium, Anaerovibrio lipolytica and Veillonella alcalescens. — J. Gen. Microbiol. 81: 69–78.
de Vries, W., van Wyck-Kapteyn, W. M. C. and Stouthamer, A. H. 1973. Generation of ATP during cytochrome-linked anaerobic electron transport in propionic acid bacteria. —J. Gen. Microbiol. 76: 31–41.
White, D. C. 1966. The obligatory involvement of the electron transport system in the catabolic metabolism of Haemophilus parainfluenzae. — Antonie van Leeuwenhoek 32: 139–158.
White, D. C. 1967. Effect of glucose on the formation of the membrane-bound electron transport system in Haemophilus parainfluenzae. — J. Bacteriol. 93: 567–573.
White, D. C. and Sinclair, P. R. 1971. Branched electron transport systems in bacteria. Adv. Microbiol. Physiol. 5: 173–211.
Wood, W. A. 1971. Assay of enzymes representative of metabolic pathways, p. 411–424. In J. R. Norris and D. W. Ribbons, (eds.), Methods in Microbiology, Vol. 6A. — Academic Press, New York-London.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holländer, R. Energy metabolism of some representatives of the Haemophilus group. Antonie van Leeuwenhoek 42, 429–444 (1976). https://doi.org/10.1007/BF00410174
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00410174