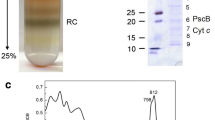
Abstract
Physico-chemical properties of homogeneous preparations of soluble and membrane-bound hydrogenases from the purple sulfur bacterium Thiocapsa roseopersicina BBS and membrane-bound hydrogenase of Rhodopseudomonas capsulata, strain B10 have been studied. Compared to the enzymes from other sources, the hydrogenase of Thiocapsa roseopersicina is more stable to O2 and products of its reduction (O -2 , H2O2), temperature and a number of other factors of the medium. A natural electron donor for T. roseopersicina hydrogenase is a low-potential cytochrome C′3, while the natural electron acceptors for hydrogenases of R. capsulata, T. roseopersicina, Ectothiorhodospira shaposhnikovii and Anabaena cylindrica are cytochromes of groups “c” and “b”.
In different phototrophs, synthesis of hydrogenase can be inhibited by the presence of high concentrations of O2. In some microorganisms (e.g. Rhodopseudomonas capsulata, strain B10) the repressing effect on hydrogenase formation is also exhibited by organic compounds. H2 may not necessarily be present for hydrogenase synthesis by purple bacteria, but its presence may considerable increase the level of the enzyme.
Similar content being viewed by others
Abbreviations
- SDS:
-
sodium dodecylsulfate
- Hipip:
-
high-potential iron — sulfur protein
- R :
-
Rhodopseudomonas
- T :
-
Thiocapsa
- Rh :
-
Rhodospirillum
- C :
-
Chromatium
References
Adams MWW, Mortenson LE, Chen J-S (1980) Hydrogenase. Biochim Biophys Acta 594:105–176
Albracht SPJ, Graf E-G, Thauer RK (1982) The EPR properties of nickel in hydrogenase from Methanobacterium thermoautotrophicum. FEBS Lett 140:311–313
Arp DJ, Burris RH (1979) Purification and properties of the particulate hydrogenase from the bacteriodes soybean root nodules. Biochim Biophys Acta 570:221–230
Berlier YM, Lespinat PA (1980) Mass-spectrometrical kinetic studies of the nitrogenase and hydrogenase activities in vivo cultures of Azospirillum brasilense. Arch Microbiol 125:67–72
Bowien B, Schlegel HG (1981) Physiology and biochemistry of aerobic hydrogen-oxidizing bacteria. Annu Rev Microbiol 35:405–452
Cammack R, Patil D, Aquirre R, Hatchkian EE (1982) Redox properties of the ESR-detectable nickel in hydrogenase from Desulfovibrio gigas. FEBS Lett 142:289–292
Chen J-S (1978) Structure and function of two hydrogenase from dinitrogen-fixing bacterium Clostridium pasteurianum. In: Schlegel HG, Schneider K (eds) Hydrogenases: Their Catalytic Activity, Structure and Function, Goltze E, Göttingen, pp 57–81
Colbeau A, Vignais PM (1981) The membrane-bound hydrogenase of Rh. capsulata: stability and catalytic properties. Biochim Biophys Acta 662:271–284
Colbeau A, Chabert F, Vignais PM (1978) Hydrogenase activity in Rhodopseudomonas capsulata. Stability and stabilization of the solubilized enzyme. In: Schlegel HG, Schneider K (eds) Hydrogenases: Their Catalytic Activity, Structure and Function, Goltze E, Göttingen, pp 183–198
Friedrich CG (1982) Derepression of hydrogenase during limitation of electron donors and derepression of ribulose-bisphosphate carboxylase during carbon limitation of Alcaligenes eutrophus. J Bacteriol 152:42–48
Friedrich CG, Schneider K, Friedrich B (1982) Nickel in the catalytically active hydrogenase of Alcaligenes eutrophus. J Bacteriol 152:42–48
Fuchs G, Moll J, Scherer P, Thauer R (1978) Activity, acceptor specificity and function of hydrogenase in Methanobacterium thermoautotrophicum. In: Schlegel HG, Schneider K (eds) Hydrogenases: Their Catalytic Activity, Structure and Function. Goltze, Göttingen, pp 83–92
Gitlitz PH, Krasna AJ (1975) Structural and catalytical properties of hydrogenase from Chromatium. Biochemistry 14:2561–2567
Gogotov IN (1973) Hydrogen metabolism and nitrogen fixation in phototrophic bacteria. In: Abstracts of Symp. on Procaryotic Photosyn. Organisms, Freiburg, pp 118–131
Gogotov IN (1978) Relationships in hydrogen metabolism between hydrogenase and nitrogenase in phototrophic bacteria. Biochimie 60:267–275
Gogotov IN (1979) Hydrogenases of microorganisms. Uspekhi mikrobiologii 14:3–27
Gogotov IN (1980) Microorganisms as hydrogen and hydrogenase producers. In: Wingard LB, Berezin IV (eds) Enzyme Enginering: Future Directions, Plenum Press, NY-L-W, pp 321–337
Gogotov IN (1984) Hydrogen production by gron cultures, cell suspension and polyenzyme systems of phototrophic bacteria. In: Berezin IV et al. (eds) Solar Energy Bioconversion. Pushchino, pp 75–85
Gogotov IN, Zorin NA, Bogorov LV (1974) Metabolism of hydrogen and nitrogen fixation capacity of Thiocapsa roseopersicina Mikrobiologiya 43:5–10
Gogotov IN, Zorin NA, Serebriakova LT, Kondratieva EN (1978) The properties of hydrogenase from Thiocapsa roseopersicina Biochim Biophys Acta 523:385–393
Hatchikian EC, Bruschi M, Le Gall J (1978) Characterization on the periplasmic hydrogenase from Desulfovibrio gigas. Biochem Biophys Res Communs 82:451–461
Klemme L-H (1968) Untersuchungen zur Photoautotrophic mit Molekularen Wasserstoff bei neuisolierten schwefelfreien Purpurbakterien. Arch Mikrobiol 64:29–42
Kondratieva EN, Gogotov IN (1981) Molecular hydrogen in the metabolism of microorganisms. Moscow, Nauka, pp 342
Kondratieva EN, Gogotov IN (1983) Production of molecular hydrogen in microorganisms. In: Adv Biochem Eng Fiechter A (ed), Springer, v. 28, B.-H.-N.Y., pp 139–191
Korsunsky OF, Smolygina LD, Laurinavichene TV, Gogotov IN (1982) Properties and functions of low-potential cytochrome c3 from Thiocapsa roseopersicina. Biokhimiya 47:355–360
Krasna AI (1980) Regulation of hydrogenase activity in Enterobacteria. J Bacteriol 144:1094–1097
Kulakova SM, Yakunin AF, Gogotov IN (1982) Stability of purple bacteria hydrogenase and nitrogenase to the inactivating action of oxygen and the products of its reduction. Prikl biokhim i mikrobiol 18:324–330
Maier RJ, Hanus FJ, Evans HJ (1979) Rgulation of hydrogenase in Rhizobium japonicum. J Bacteriol 137:824–829
Meyer J, Kelley BC, Vignais PM (1978) Nitrogen fixation and hydrogen metabolism in photosynthetic bacteria. Biochimie 60:245–260
Mortenson LE, Chen J-S (1974) Hydrogenase. In: Heiland JB (ed) Microbiol Iron Metabolism. Acad Press, New York, pp 231–282
Partridge CDP, Walker CC, Yates MG, Postgate JR (1980) The relationship between hydrogenase and nitrogenase in Azotobacter chroococcum: effect of N-source on hydrogenase activity. J Gen Microbiol 119:313–319
Quist RC, Stokes JL (1972) Comparative effect of temperature on the induced synthesis of hydrogenase and enzymes of the benzoate oxidation system in psychrophilic and mesophilic bacteria. Can J Microbiol 18:1233–1239
Schlegel HG, Eberhardt U (1972) Regulatory phenomena in the metabolism of knallgasbacteria. Adv Microbiol Physiol 7:205–243
Strekas T, Antanaitis BC, Krasna AJ (1980) Characterization and stability of hydrogenase from Chromatium. Biochim Biophys Acta 116:1–9
Tsygankov AA, Gogotov IN (1979) Continuous cultivation of phototrophic bacteria. Prikl biokhim i mikrobiol 5:665–670
Tsygankov AA, Yakunin AF, Gogotov IN (1982a) Hydrogenase activity of Rhodopseudomonas capsulata during growth on organic media. Mikrobiologiya 51:533–537
Tsygankov AA, Pavlova EA, Gogotov IN (1982b) Activity of some enzymes involved in H2 metabolism of Rhodopseudomonas capsulata depending on growth conditions of the cultures. Mikrobiologiya 51:188–193
Van der Werf A, Yates MG (1978) Hydrogenase from nitrogenfixing Azotobacter chroococcum. In: Schlegel HG, Schneider K (eds) Hydrogenases: Their Catalytic Activity, Structure and Function. Goltze, Göttingen, pp 307–326
Yagi T, Endo A, Tsuji K (1978) Properties of hydrogenase from particulate fraction of Desulfovibrio vulgaris. In: Schlegel HG, Schneider K (eds) Hydrogenases: Their Catalytic Activity, Structure and Function. Goltze, Göttingen, pp 107–124
Zorin NA, Gogotov IN (1980) Purification and properties of cytochrome c552 from purple sulfur bacterium Thiocapsa roseopersicina. Biokhimiya 45:1497–1502
Zorin NA, Gogotov IN (1982) Stability of hydrogenase of purple sulfur bacterium Thiocapsa roseopersicina. Biokhimiya 47:827–833
Zorin NA, Gogotov IN (1983) High-potential iron-sulfur protein of Thiocapsa roseopersicina. Biokhimiya 48:1181–1187
Author information
Authors and Affiliations
Additional information
This paper is dedicated to Professor Dr. H.G. Schlegel in honour of his sixtieth birthday and in recognition of his great contribution in the field of physiology and biochemistry of microorganisms capable of using H2. Professor H.G. Schlegel had a profound and most fuitful influence on the progress in the research of the laboratory headed by the author
Rights and permissions
About this article
Cite this article
Gogotov, I.N. Hydrogenase of purple bacteria: properties and regulation of synthesis. Arch. Microbiol. 140, 86–90 (1984). https://doi.org/10.1007/BF00409777
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00409777