Summary
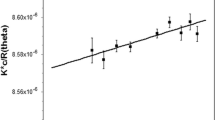
An extracellular endopolygalacturonate lyase of Cytophaga johnsonii was purified from the culture filtrate. It appeared to be homogeneous as judged by polyacrylamide gel electrophoresis at pH 8.6 as well as pH 4.3. The purified enzyme had a pH optimum around 9.0 and required Ca++ ions for its maximum activity. The apparent K mfor polygalacturonic acid was found to be 0.22%. Both paper and column chromatography indicated formation and accumulation of an unsaturated monomer along with unsaturated di-, tri-, tetra- and pentamers from polygalacturonic acid by the enzyme action, indicating that the enzyme cleaved the substrate randomly in a non-hydrolytic manner. The glycosidic linkage next to the non-reducing end of polygalacturonic acid was not resistant to attack by this enzyme unlike in other known polygalacturonate lyases.
Similar content being viewed by others
Abbreviations
- PG lyase:
-
Polygalacturonate lyase
- Tris:
-
Tris (hydroxymethyl) aminomethane
References
Albersheim, P., Neukom, H., Deuel, H.: Splitting of pectin chain molecules in neutral solutions. Arch. Biochem. 90, 46–51 (1960a).
———: Über die Bildung von ungesättigten Abbauprodukten durch ein pektinabbauendes Enzym. Helv. chim. Acta 43, 1422–1426 (1960b).
Anderson, R. L., Ordal, E. J.: Cytophaga succinicans Sp. N., a facultatively anaerobic, aquatic myxobacterium. J. Bact. 81, 130–138 (1961).
Andrews, P.: Estimation of molecular weights of proteins by Sephadex gel-filtration. Biochem. J. 91, 222–233 (1964).
Bacon, J. S. D., Edelman, J.: The carbohydrates of the Jerusalem Artichoke and other compositae. Biochem. J. 48, 114–126 (1951).
Bhat, J. V., Jayasankar, N. P., Agate, A. D., Bilimoria, M. H.: Microbial degradation of pectic substances. Biochem. Rev. India 37, 23–29 (1966).
Davis, B. J.: Disc electrophoresis. II. Method and application to human proteins. Ann. N. Y. Acad. Sci. 121, 404–427 (1964).
Edstrom, R. D., Phaff, H. J.: Eliminative cleavage of pectin and oligogalacturonide methyl esters by pectin trans-eliminase. J. biol. Chem. 239, 2409–2415 (1964).
French, D., Wild, G. M.: Correlation of carbohydrate structure with papergram mobility. J. Amer. chem. Soc. 75, 2612–2616 (1953).
Fuchs, A.: The trans-eliminative breakdown of Na-polygalacturonate by Pseudomonas fluorescens. Antonie v. Leeuwenhoek 31, 323–340 (1965).
Kamat, N. K., Bhat, J. V.: Pectin trans-eliminase activity in Cytophaga. Curr. Sci. 36, 486 (1967).
Lanning, M. C., Cohen, S. S.: The detection and estimation of 2-ketohexonic acids. J. biol. Chem. 189, 109–114 (1951).
Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J.: Protein measurement with the folin phenol reagent. J. biol. Chem. 193, 265–275 (1951).
MacMillan, J. D., Vaughn, R. H.: Purification and properties of a polygalacturonic acid trans-eliminase produced by Clostridium multiferans. Biochemistry 3, 564–572 (1964).
Moran, F., Nasuno, S., Starr, M. P.: Extracellular and intracellular polygalacturonic acid trans-eliminase of Erwinia caratovora. Arch. Biochem. 123, 298–306 (1968).
Nagel, C. W., Anderson, M. M.: Action of a bacterial trans-eliminase on normal and unsaturated oligogalacturonic acids. Arch. Biochem. 112, 322–330 (1965).
—, Vaughn, R. H.: The characteristics of a polygalacturonase produced by Bacillus polymyxa. Arch. Biochem. 93, 344–352 (1961a).
——: The degradation of oligogalacturonides by the polygalacturonase of Bacillus polymyxa. Arch. Biochem. 94, 328–332 (1961b).
Nasuno, S., Starr, M. P.: Polygalacturonic acid trans-eliminase of Xanthomonas campestris. Biochem. J. 104, 178–185 (1967).
Patel, D. S., Phaff, H. J.: On the mechanism of action of yeast endo-polygalacturonase on oligogalacturonides and their reduced and oxidised derivatives. J. biol. Chem. 234, 237–241 (1959).
Stanier, R. Y.: The Cytophaga group: A contribution to the biology of myxobacteria. Bact. Rev. 6, 143–196 (1942).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sundarraj, N., Bhat, J.V. Endo-polygalacturonate lyase of Cytophaga johnsonii . Archiv. Mikrobiol. 77, 155–164 (1971). https://doi.org/10.1007/BF00408608
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00408608