Abstract
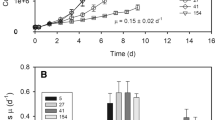
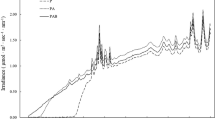
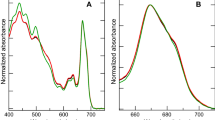
When Porphyridium cruentum cells were illuminated with high fluence rate between 1900 and 4800 μmol photons m-2s-1, a decrease in the photosynthetic activity of the cells was observed. Within the time frame of 20 min, and under the fluence rates studied, the sum of photons to be absorbed by cells (mg of chlorophyll (Chl), sufficient to initiate photoinhibition was calculated to be 9235.8 μmol. The minimal specific light absorption rate to initiate photoinhibition in P. cruentum ranges between 2.29 and 4.26 μmol photons s-1 mg-1 chl.a. There was a linear relationship between the specific rate of photoinhibition and the specific light absorption rate. A photon number of 2.56×104 μmol mg-1 chl.a photoinhibited photosynthesis instantaneously. At 15°C, no photoinhibitory effect was observed at 2300 μmol photons m-2 s-1 even after 45 min of illumination. At the other extreme of 35°C, 84% inhibition of photosynthetic activity was observed within 10 min of exposure to 2300 μmol photons m-2 s-1. Between 20 and 30°C, the photoinhibitory effect was comparable. Photoinhibited P. cruentum cells recovered readily when transferred to low light (90 μmol photons m-2 s-1) and darkness, and the specific rate of recovery was independent of the light intensity to which the cells were exposed, during the photoinhibitory treatment.
Similar content being viewed by others
Abbreviations
- Chlorophyll:
-
QL, specific light absorption rate
References
Ahern TJ, Katoh S, Sada E (1983) Arachidonic acid production by the red alga Porphyridium cruentum. Biotechnol Bioeng 225:1057–1070
Bjorkman O, Boardman NK, Anderson JM, Thorne SW, Goodchild DJ, Pyliotis NA (1972) Effect of light intensity during growth of Atriplex patula on the capacity of photosynthetic reactions, chloroplast components and structure. Carnegie Inst Washington Year b 71:115–135
Curtin ME (1985) Chemicals from the sea. Biotechnol 3:34–37
Horton P, Hague A (1986) Recovery from photoinhibition in isolated protoplasts. Annual Report of the Research Institute for Photosynthesis, University of Sheffield 4-6
Iehana M (1987) Kinetic analysis of the growth of Spiruline sp in bath culture. J Ferment Technol 65:267–275
Jones LW, Kok B (1966a) Photoinhibition of chloroplast reactions. I. Kinetics and action spectra. Plant Physiol 41:1037–1043
Jones LW, Kok B (1966b) Photoinhibition of chloroplast reactions. II. Multiple effects. Plant Physiol 41:1044–1049
Jones RF, Speer HL, Kury W (1983) Studies on the growth of the red alga Porphyridium cruentum. Plant Physiol 16:636–643
Kok B (1956) On the inhibition of photosynthesis by intense light. Biochim Biophys Acta 21:234–244
Krinsky NI (1976) Cellular damage initiated by visible light. In: Gray TRG, Postgate JR (eds) The survival of vegetative microbes Cambridge University Press, Cambridge, pp 209–239
Kyle DJ, Ohad I (1986) The mechanisms of photoinhibition in higher plants and green algae. In: Staehelin LA, Arntzen CJ (eds) Encylopedia of plant physiology, Photosynthesis III. vol 19. Springer, Berlin Heidelberg New York, pp 468–475
Kyle DJ, Arntzen CJ, Ohad I (1983) The herbicide-binding 32 kD polypeptide is the primary site of photoinhibition damage. Plant Physiol Suppl 72 :52
Kyle DJ, Ohad I, Arntzen CJ (1984) Membrane protein damage and repair. I. Selective loss of quinone protein function in chloroplast membranes. Proc Natl Acad Sci USA 81:4070–4074
Lee YK, Pirt SJ (1981) Energetics of photosynthetic algal growth: Influence of intermittent illumination in short cycle. J Gen Microbiol 124:43–52
Lee YK, Tan HM (1988) Effect of temperature, light intensity and dilution rate on the cellular composition of red alga Porphyridium cruentum in light-limited chemostat cultures. MIRCEN J Appl Microbiol Biotechnol 1: (in press)
Matto AK, Hoffman FH, Marder JB, Edelman M (1984) Regulation of protein metabolism: Coupling of photosynthetic electron transport to in vivo degradation of rapidly metabolized 32 kD protein of the chloroplast membranes. Proc Natl Acad Sci USA 81:1380–1384
Ogren E, Oquist S (1984) Photoinhibition of photosynthesis in Lemna Gibba as induced by the interaction between light and temperature. Plant Physiol 62:193–200
Percival E, Foyle RAJ (1979) The extracellular polysaccharides of Porphyridium cruentum and Porphyridium aerugineum. Carbohydrate Res 72:165–176
Powels SB (1984) Photoinhibition of photosynthesis induced by visible light. Ann Rev Plant Physiol 35:15–44
Powels SB, Thorne SW (1981) Effect of high-light treatments in inducing photoinhibition of photosynthesis in intact leaves of low-light growth Phaseolus vulgaris and Lastreopsis microsora. Planta 152:471–477
Richardson K, Beardall J, Raven J (1983) Adaptation of unicellular algae to irradiance: An analysis of strategies. New Phytol 93:157–191
Samuelsson G, Lönneborg AL, Rosenquist E, Gustafsson P, Oquist G (1985) Photoinhibition and reactivation of photosynthesis in the cyanobacteria Anacystis nidulans. Plant Physiol 79:992–995
Samuelsson G, Lönneborg A, Gustafsson P, Oquist G (1987) The susceptibility of photosynthesis to photoinhibition and the capacity of recovery in high and low light grown cyanobacteria Anacystis nidulans. Plant Physiol 83:438–441
Thepenier C, Gudin C (1985) Studies on optimal conditions for polysaccharide production by Porphyridium cruentum. MIRCEN J Appl Microbiol Biotechnol 1:257–268
Trebst A, Draber W (1986) Inhibitors or photosystem II and the topology of the herbicide and QB binding polypeptide in the thylakoid membrane. Photosynthetic Research 10:381–392
Vonshak A, Guy R (1988) Photoinhibition and productivity of Spirulina strains. In: Stadler T (ed) Proceedings of the 4th International Meeting of the Societe pour l'Algologie Appliquee, Villeneuve d'Ascq, France. Elsevier Applied Science, Essex (in press)
Vonshak A, Cohon Z, Richmond A (1985) The feasiblity of mass cultivation of Porphyridium. Biomass 8:13–25
Vonshak A, Guy R, Poplawsky R, Ohad I (1988) Photoinhibition and its recovery in two strains of the cyanobacteria Spirulina platensis. Plant Cell Physiol (in press)
Wettern M, Galling G (1985) Degradation of the 32 kDa thylakoid membrane polypeptide of Chlamydomonas reinhardtii Y-1. Planta 166:474–482
Author information
Authors and Affiliations
Additional information
Publication No. 28 of the Microalgal Biotechnology Laboratory
Rights and permissions
About this article
Cite this article
Lee, Y.K., Vonshak, A. The kinetics of photoinhibition and its recovery in the red alga Porphyridium cruentum . Arch. Microbiol. 150, 529–533 (1988). https://doi.org/10.1007/BF00408244
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00408244