Abstract
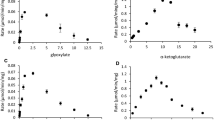
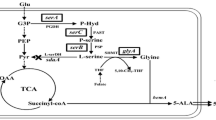
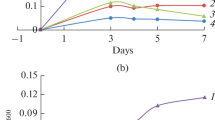
Peptococcus glycinophilus and P. magnus (P. variabilis) utilized only glycine-containing compounds for growth and required selenium compounds for the fermentation of glycine in an optimized medium. Under these conditions an active glycine reductase was expressed in vivo as demonstrated for both species by tracer experiments and additionally in vitro for P. glycinophilus in cell extracts. It is concluded that the availability of selenium determines the fermentation pathway of glycine. In the presence of selenite glycine is converted via the glycine reductase rather than the hitherto known glycine-serine-pyruvate interconversion. The molar growth yield of P. magnus was determined to be 8.9 g of dry weight per mol of glycine. The significance of the low vitamin B12 content and a low carbon monoxide dehydrogenase activity in P. glycinophilus for the reduction of CO2 to acetate is discussed.
Similar content being viewed by others
References
Andreesen JR (1980) Role of selenium, molybdenum and tungsten in anaerobes. In: Gottschalk G, Pfennig N, Werner H (eds) Anaerobes and anaerobic infections. Fischer, Stuttgart, pp 31–40
Baginsky ML, Huennekens FM (1966) Electron transport function of a heat-stable protein and a flavoprotein in the oxidative decarboxylation of glycine by Peptococcus glycinophilus. Biochem Biophys Res Commun 23:600–605
Baginsky ML, Huennekens FM (1967) Further studies on the electron transport proteins involved in the oxidative decarboxylation of glycine. Arch Biochem Biophys 120:703–711
Barker HA, Volcani BE, Cardon BP (1948) Tracer experiments on the mechanism of glycine fermentation by Diplococcus glycinophilus. J Biol Chem 173:803–804
Beisenherz G, Boltze HJ, Bücher T, Czok R, Garbade KH, Meyer-Arendt E, Pfleiderer G (1953) Diphosphofructose-Aldolase, Phosphoglyceraldehyd-Dehydrogenase, Milchsäure-Dehydrogenase, Glycerophosphat-Dehydrogenase und Pyruvat-Kinase aus Kaninchenmuskulatur in einem Arbeitsgang. Z Naturforsch 8b:555–577
Braun K (1979) Untersuchungen zum autotrophen, heterotrophen und mixotrophen Wachstum von Acetobacterium woodii and Clostridium aceticum. Ph. D. Thesis Univ Göttingen
Cardon BP, Barker HA (1946) Two new amino-acid-fermentating bacteria, Clostridium propionicum and Diplococcus glycinophilus. J Bacteriol 52:629–634
Cardon BP, Barker HA (1947) Amino acid fermentation by Clostridium propionicum and Diplococcus glycinophilus. Arch Biochem 12:165–180
Champion AB, Rabinowitz JC (1977) Ferredoxin and formyltetrahydrofolate synthetase: comparative studies with Clostridium acidiurici, Clostridium cylindrosporum, and newly isolated anaerobic uric acidfermenting strains. J Bacteriol 132:1003–1020
Clark JE, Ragsdale SW, Ljungdahl LG, Wiegel J (1982) Levels of enzymes involved in the synthesis of acetate from CO2 in Clostridium thermoautotrophicum. J Bacteriol 151:507–509
Costilow RN (1977) Selenium requirement for the growth of Clostridium sporogenes with glycine as the oxidant in Stickland reaction systems. J Bacteriol 131:366–368
Diekert GB, Thauer RK (1978) Carbon monoxide oxidation by Clostridium thermoaceticum and Clostridium formicoaceticum. J Bacteriol 136:597–606
Dorn M, Andreesen JR, Gottschalk G (1978) Fermentation of fumarate and l-malate by Clostridium formicoaceticum. J Bacteriol 133:26–32
Douglas HC (1951) Glycine fermentation by nongas forming anaerobic micrococci. J Bacteriol 62:517–518
Douglas HC (1957) Genus VI. Peptococcus Kluyver and van Niel, 1936. In: Breed, RS, Murray RGD, Smith NR (eds) Bergey's manual of determinative bacteriology, 7th ed. Williams & Wilkins Co, Baltimore, pp 474–480
Drake HL, Hu S-I, Wood HG (1981) Purification of five components from Clostridium thermoaceticum which catalyze synthesis of acetate from pyruvate and methyltetrahydrofolate. J Biol Chem 256:11137–11144
Dürre P, Andersch W, Andreesen JR (1981) Isolation and characterization of an adenine-utilizing, anaerobic sporeformer, Clostridium purinolyticum sp. nov. Int J Syst Bacteriol 31:184–194
Dürre P, Andreesen JR (1982a) Selenium-dependent growth and glycine fermentation by Clostridium purinolyticum. J Gen Microbiol 128:1457–1466
Dürre P, Andreesen JR (1982b) Pathway of carbon dioxide reduction to acetate without a net energy requirement in Clostridium purinolyticum. FEMS Microbiol Lett 15:51–56
Dürre P, Andreesen JR (1983) Purine and glycine metabolism by purinolytic clostridia. J Bacteriol 154
Foubert EL, Douglas HC (1948) Studies on the anaerobic micrococci. I. Taxonomic considerations. J Bacteriol 56:25–34
Guttman HN (1963) Vitamin B12 and congeners. In: Kavanagh F (ed) Analytical microbiology. Academic Press, New York, pp 527–550
Hare R (1967) The anaerobic cocci. In: Waterson HP (ed) Recent advances in medical microbiology. Little, Brown and Comp, Boston, pp 284–317
Holdeman LV, Moore WEC (1972) Anaerobe laboratory manual, 2nd edn. Anaerobe Laboratory Virginia Polytechnic Institute and State University, Blacksburg
Hu S-I, Drake HL, Wood HG (1982) Synthesis of acetyl coenzyme A from carbon monoxide, methyltetrahydrofolate, and coenzyme A by enzymes from Clostridium thermoaceticum. J Bacteriol 149:440–448
Kaplan A (1969) The determination of urea, ammonia, and urease. Methods Biochem Anal 17:311–324
Klein SM, Sagers RD (1962) Intermediary metabolism of Diplococcus glycinophilus. II. Enzymes of the acetate-generating system. J Bacteriol 83:121–126
Klein SM, Sagers RD (1966a) Glycine metabolism. I. Properties of the system catalyzing the exchange of bicarbonate with the carboxyl group of glycine in Peptococcus glycinophilus. J Biol Chem 241:197–205
Klein SM, Sagers RD (1966b) Glycine metabolism. II. Kinetic and optical studies on the glycine decarboxylase system from Peptococcus glycinophilus. J Biol Chem 241:206–209
Klein SM, Sagers RD (1967a) Glycine metabolism. III. A flavin linked dehydrogenase associated with the glycine cleavage system in Peptococcus glycinophilus. J Biol Chem 242:297–300
Klein SM, Sagers RD (1967b) Glycine metabolism. IV. Effect of borohydride reduction on the pyridoxal phosphate-containing glycine decarboxylase from Peptococcus glycinophilus. J Biol Chem 242:301–305
Lang E, Lang H (1972) Spezifische Farbreaktion zum direkten Nachweis der Ameisensäure. Fresenius Z Anal Chem 260:8–10
Ljungdahl LG, Wood HG (1982) Acetate biosynthesis. In: Dolpin D (ed) B12. John Wiley & Sons, New York, pp 165–202
Meyer O, Schlegel HG (1978) Reisolation of the carbon monoxide utilizing hydrogen bacterium Pseudomonas carboxydovorans (Kistner) comb. nov. Arch Microbiol 118:35–43
National Research Council (1976) Selenium. National Academy of Sciences, Washington D.C.
Pfennig N, Widdel F, Trüper HG (1981) The dissimilatory sulfatereducing bacteria. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The prokaryotes. A handbook on habitats, isolation, and identification of bacteria, vol 1. Springer, Berlin Heidelberg New York, pp 926–940
Robinson JR, Klein SM, Sagers RD (1973) Glycine metabolism. Lipoic acid as the prosthetic group in the electron transfer protein P2 from Peptococcus glycinophilus. J Biol Chem 248:5319–5323
Sagers RD, Gunsalus IC (1961) Intermediary metabolism of Diplococcus glycinophilus. I. Glycine cleavage and one-carbon interconversions. J Bacteriol 81:541–549
Sagers RD, Dalling DK, Horton WJ, Grant DM (1977) C-13 NMR observations on the conversion of glycine to acetate. Abstr Annu Meet ASM, K 110, p 204
Sardesai VM, Provido HS (1970) The determination of glycine in biological fluids. Clin Chim Acta 29:67–71
Schulman M, Parker D, Ljungdahl LG, Wood HG (1972) Total synthesis of acetate from CO2. V. Determination by mass analysis of the different types of acetate formed from 13CO2 by heterotrophic bacteria. J Bacteriol 109:633–644
Singh JV, Khanna SK, Singh GH (1978) Effect of manganese on ninhydrin color development by amino acids. Anal Biochem 85:581–585
Skerman VBD, McGowan V, Sneath PHA (1980) Approved lists of bacterial names. Int J Syst Bacteriol 30:225–420
Stadtman TC (1970) Glycine reductase system (Clostridium). In: Tabor H, Tabor CW (eds) Methods in enzymology, vol 17 A. Academic Press, New York, pp 959–966
Stickland LH (1951) The determination of small quantities of bacteria by means of the biuret reaction. J Gen Microbiol 5:698–703
Stouthamer AH (1979) The search for correlation between theoretical and experimental growth yields. In: Quayle JR (ed) Microbial biochemistry, international review of biochemistry, vol 21. University Park Press, Baltimore, pp 1–47
Tanner RS, Stackebrandt E, Fox GE, Gupta R, Magrum LJ, Woese CR (1982) A phylogenetic analysis of anaerobic eubacteria capable of synthesizing acetate from carbon dioxide. Curr Microbiol 7:127–132
Turner DC, Stadtman TC (1973) Purification of protein components of the clostridial glycine reductase system and characterization of protein A as a selenoprotein. Arch Biochem Biophys 154:366–381
Waber JL, Wood HG (1979) Mechanism of acetate synthesis from CO2 by Clostridium acidiurici. J Bacteriol 140:468–478
Weiss N (1981) Cell wall structure of anaerobic cocci. Rev Inst Pasteur Lyon 14:53–59
West SEH, Holdeman LV (1973) Placement of the name Peptococcus anaerobius (Hamm) Douglas on the list of nomina rejicienda. Int J Syst Bacteriol 23:283–289
Wildy P, Hare R (1953) The effect of fatty acids on the growth, metabolism and morphology of the anaerobic cocci. J Gen Microbiol 9:216–225
Yamamoto I, Saiki T, Lin SM, Ljungdahl LG (1983) Purification and properties of NADP-dependent formate dehydrogenase from Clostridium thermoaceticum, a tungsten-selenium iron protein. J Biol Chem (in press)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dürre, P., Spahr, R. & Andreesen, J.R. Glycine fermentation via a glycine reductase in Peptococcus glycinophilus and Peptococcus magnus . Arch. Microbiol. 134, 127–135 (1983). https://doi.org/10.1007/BF00407945
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00407945