Abstract
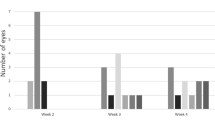
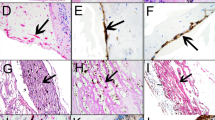
•Background: This study examined the relationship between growth factor expression and cellular proliferation during the evolution of traumatic tractional retinal detachment (TRD) in a rabbit model. •Methods: TRD was induced in 15 pigmented rabbits by treating the inferior retina with cryopexy and making a scleral incision superiorly. Sections from varied time points were stained in the same assay with mouse monoclonal antibodies (MAb) specific for basic fibroblastic growth factor (bFGF) and platelet-derived growth factor (PDGF-BB/AB). • Results: Initially, the eyes exhibited intense vitritis; discrete membranes were present at 7 days and progressed to tractional retinal detachment at 17 and 28 days, after which there was no clinical change. At 6 and 24 h, bFGF, PDGF, and proliferating cell nuclear antigen (PCNA) were not detectable in membranes or wound sites (except for PDGF-positive in flammatory cells). On days 7, 17, 28, and 52, bFGF and PDGF were readily detectable in most membranes. Cellular proliferation as detected by PCNA staining was also present on days 7, 17, and 28, but was absent by day 52 despite growth factor staining. At all times, PCNA staining, which was most intense at the wound site, showed only limited correlation with staining for either growth factor for individual cells. Müller cells stained positively for PDGF-BB/AB in 13 of the 15 TRD eyes, but in none of the normal eyes. • Conclusions: Since cellular proliferation correlated incompletely with the staining for bFGF and PDGF, these growth factors may not account exclusively for cellular proliferation within the membrane. Their distribution, however, including PDGF staining of Müller cells and bFGF staining at the vitreous-membrane interface, suggests that they may have roles in the pathogenesis of TRD.
Similar content being viewed by others
References
Bohlen P, Esch F, Baird A, Gospodarowicz D (1985) Acidic fibroblast growth factor from bovine brain. Amino terminal sequence and comparison to basic fibroblast growth factor. EMBO J 4:1951–1956
Bryan JA III, Campochiaro PA (1986) A retinal pigment epithelial cellderived growth factor(s). Arch Ophthalmol 104:422–425
Buntrock P, Jentzsch KD, Heder G (1982) Stimulation of wound healing using brain extract with FGF activity. I. Quantitative and biochemical studies into the formation of granulation tissue. Exp Pathol 21:46–53
Campochiaro PA, Gaskin HC, Vinores SA (1987) Retinal cryopexy stimulates traction retinal detachment formation in the presence of an ocular wound. Arch Ophthalmol 105:1567–1570
Campochiaro PA, Sugg R, Grotendorst G, Hjelmeland LM (1989) Retinal pigment epithelial cells produce PDGF-like proteins and secrete them into their media. Exp Eye Res 49:217–227
Casscells W, Speir E, Sasse J, et al, (1990) Isolation, characterization, and localization of heparin-binding growth factors in the heart. J Clin Invest 85:433–441
Clark RAF, Folkvord JM, Hart CE, Murray MJ, McPherson JM (1989) Platelet isoforms of platelet-derived growth factor stimulate fibroblasts to contract collagen matrices. J Clin Invest 84:1036–1040
Connor TB Jr, Roberts AB, Sporn MB, et al, (1989) Correlation of fibrosis and transforming growth factor-β type 2 levels in the eye. J Clin Invest 83:1661–1666
De Juan E, Dickson JS, Hjelmeland L (1988) Serum is chemotactic for retina-derived glial cells. Arch Ophthalmol 106:986–990
Erickson PA, Fisher SK, Guérin CJ, et al, (1987) Glial fibrillary acidic protein increases in Müller cells after retinal detachment. Exp Eye Res 44:37–48
Fairman MP (1990) DNA polymerase δ/PCNA: actions and interactions. J Cell Sci 95:1–4
Gospodarowicz D (1989) Fibroblast growth factor. Crit Rev Oncogenesis 1:1–26
Guérin CJ, Anderson D, Fisher SK (1990) Changes in intermediate filament immunolabeling occur in response to retinal detachment and reattachment in primates. Invest Ophthalmol Vis Sci 31:1474–1482
Hall PA, Levison DA, Woods AL, et al. (1990) Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol 162:285–294
Hart CE, Bailey M, Curtis DA, et al. (1990) Purification of PDGF-AB and PDGF-BB from human platelet extracts and identification of all 3 PDGF dimers in human platelets. Biochemistry 29:166–172
Harvey AK, Roberge F, Hjelmeland LM (1987) Chemotaxis of rat retinal glia to growth factors found in repairing wounds. Invest Ophthalmol Vis Sci 28:1092–1099
Jeanny JC, Fayaein N, Moenner M, Chevallier B, Barritault D, Courtois Y (1987) Specific fixation of bovine brain and retinal acidic and basic fibroblast growth factors to mouse embryonic eye basement membranes. Exp Cell Res 171:63
Leschey KH, Hackett SF, Singer J, Campochiaro PA (1990) Growth factor responsiveness of human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 31:839–846
Okada M, Matsumura M, Ogino N, Honda Y (1990) Müller cells in detached human retina express glial fibrillary acidic protein and vimentin. Graefe's Arch Clin Exp Ophthalmol 228:467–464
Planck SR, Andresevic J, Chen JC, et al. (1991) Expression of growth factor mRNA in rabbit PVR model systems. Curr Eye Res 11:1031–1039
Powers MR, Planck SR, Wilson D, Hart CE, Rosenbaum IT (1993) Immunolocalization of platelet-derived growth factor in the neonatal rat retina (abstract). Invest Ophthalmol Vis Sci 34 [Suppl]:1587
Takahashi H, Stratton GM, Parsons PG (1991) Determination of proliferating fractions in malignant melanomas by anti-PCNA cyclin monoclonal antibody. Histopathology 18:221–227
Whitby DJ, Ferguson MW (1991) Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol 147:207–215
Yeo HJ, Sadeghi J, Campochiaro PA, Green WR, Glaser BM (1986) Intravitreous fibronectin and plateletderived growth factor. Arch Ophthalmol 104:417–421
Yoshida A, Ishiguro S, Tamai M (1993) Expression of glial fibrillary acidic protein in rabbit Müller cells after lensectomy-vitrectomy. Invest Ophthalmol Vis Sci 34:3154–3160
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Westra, I., Robbins, S.G., Wilson, D.J. et al. Time course of growth factor staining in a rabbit model of traumatic tractional retinal detachment. Graefe's Arch Clin Exp Ophthalmol 233, 573–581 (1995). https://doi.org/10.1007/BF00404709
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00404709