Summary
Although formation of DNA adducts has been postulated for several halomethanes, no chemical identification of such adducts has been performed so far. There is, however, evidence that methyl chloride does not act biologically as a DNA methylating agent. 1,2-Dichloroethane and 1,2-dibromoethane are activated through conjugation with glutathione. There is some evidence for formation on an N-7 adduct of guanine which carries an ethyl-S-cysteinyl moiety.
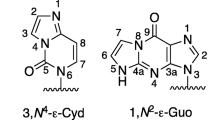
Extensive work has been published on adducts of vinyl chloride, both in vitro and in vivo. The major DNA adduct is 7-(2-oxoethyl)guanine; a minor adduct appears to be N2,3-ethenoguanine. Other “etheno” adducts, i.e., 1,N6-ethenoadenine and 3,N4-ethenocytosine, are readily formed with DNA, vinyl chloride, and a metabolizing system in vitro and with RNA in vivo, but are usually not detected as DNA adducts in vivo.
The data on DNA alkylation by vinyl chloride (and vinyl bromide) metabolites are compared with those of structurally related compounds (acrylonitrile, vinyl acetate, vinyl carbamate).
Similar content being viewed by others
References
Banerjee S, Van Duuren BL (1978) Covalent binding of the carcinogen trichloroethylene to hepatic microsomal proteins and to exogenous DNA in vitro. Cancer Res 38:776–780
Banerjee S, Van Duuren BL, Kline SA (1979) Interaction of potential metabolites of the carcinogen ethylene dibromide with protein and DNA in vitro. Biochem Biophys Res Commun 90:1214–1220
Banerjee S, Van Duuren BL, Oruambo FI (1980) Microsome mediated covalent binding of 1,2-dichloroethane to lung microsomal protein and salmon sperm DNA. Cancer Res 30:2170–2173
Barbin A, Bresil H, Croisy A, Jacquignon P, Malaveille C, Montesano R, Bartsch H (1975) Liver microsome mediated formation of alkylating agents from vinyl bromide and vinyl chloride. Biochem Biophys Res Commun 67:596–603
Barbin A, Laib RJ, Bartsch H (1985) Lack of miscoding properties of 7-(2-oxoethyl)guanine, the major vinyl chloride-DNA adduct. Cancer Res 45:2440–2444
Bartsch H, Terracini B, Malaveille C, Tomatis L, Wahrendorf J, Brun G, Dodet B (1983) Quantitative comparison of carcinogenicity, mutagenicity, and electrophilicity of 10 direct-acting alkylating agents and of the initial O6:7-alkylguanine ratio in DNA with carcinogenic potency in rodents. Mutat Res 110:181–219
Bergman K (1982) Reactions of vinyl chloride with RNA and DNA of various mouse tissues in vivo. Arch Toxicol 49:117–129
Bergman K (1983) Interactions of trichloroethylene with DNA in vitro and with RNA and DNA of various mouse tissues in vivo. Arch Toxicol 54:181–193
van Bladeren PJ (1981) The dual role of glutathione conjugation in the biotransformation of xenobitotics. Thesis, University Leiden, The Netherlands
Di Renzo AB, Gandolfi AJ, Sipes IG (1982) Microsomal bioactivation and covalent binding of aliphatic halides to DNA. Toxicology Lett 11:243–252
Djalali-Behzad G, Hussain S, Osterman-Golkar S, Segerbäck D (1981) Estimation of genetic risk of alkylating agents. VI. Exposure of mice and bacteria to methyl bromide. Mutat Res 84:1–9
Farooqui MYH, Ahmed AE (1983) In vivo interactions of acrylonitrile with macromolecules in rats. Chem Biol Interact 47:363–371
Geiger LE, Hogy LL, Guengerich FP (1983) Metabolism of acrylonitrile by isolated rat hepatocytes. Cancer Res 43:3080–3087
Gomez MID, Castro JA (1980) Covalent binding of chloroform metabolites to nuclear proteins-no evidence for binding to nucleic acids. Cancer Lett 9:213–218
Gomez MID, Castro JA (1980a) Covalent binding of carbon tetrachloride metabolites to liver nuclear DNA, proteins and lipids. Toxicol Appl Pharmacol 56:199–206
Gomez MID, Castro JA (1981) Reaction of trichloromethyl free radicals with deoxyribonucleic acid bases. Res Comm Chem Pathol Pharmacol 32:147–153
Green T, Hathway DE (1978) Interactions of vinyl chloride with rat liver DNA in vivo. Chem Biol Interact 22:211–224
Guengerich FP, Mason PS, Stoff WT, Fox TR, Watanabe PG (1981) Roles of 2-haloethylene oxides and 2-haloacetaldehydes derived from vinyl bromide and vinyl chloride in irreversible binding to protein and DNA. Cancer Res 41:4391–4398
Gwinner LM, Laib RJ, Filser JG, Bolt HM (1983) Evidence of chloroethylene oxide being the reactive metabolite of vinyl chloride towards DNA: comparative studies with 2,2′-dichloroethyl ether. Carcinogenesis 4:1483–1486
Hall JA, Saffhill R, Green T, Hathway DE (1981) The induction of errors during in vitro DNA synthesis following chloroacetaldehyde-treatment of poly (dA-dT) and poly (dC-dG) templates. Carcinogenesis 2:141–146
Harders I, Kunz W, Uehleke H, Werner B (1976) Irreversible binding of 14CCl4-metabolites to reduced phosphopyridine nucleotides in vivo and in vitro. Naunyn-Schmiedeberg's Arch Pharmacol (Suppl) 293:R65
Hathway DE (1977) Comparative mammalian metabolism of vinyl chloride and vinylidene chloride in relation to oncogenic potentia. Environ Health Perspect 21:55–59
Kornbrust DJ, Bus JS, Doerjer G, Swenberg JA (1982) Association of inhaled 14C-methyl chloride with macromolecules from various rat tissues. Toxicol Appl Pharmacol 65:122–134
Laib RJ, Gwinner LM, Bolt HM (1981) DNA alkylation by vinyl chloride metabolites: etheno derivatives or 7-alkylation of guanine? Chem Biol Interact 37:219–231
Laib RJ (1986) The role of cyclic base adducts in vinyl chloride induced carcinogenesis: studies on nucleic acid alkylation in vivo. In: Bartsch H, Singer B (eds) The role of cyclic nucleic acid adducts in carcinogenesis and mutagenesis. International Agency for Research on Cancer, Lyon
Mazzullo M, Colacci A, Grilli S, Prodi G, Arfellini G (1984) In vivo and in vitro binding of epichlorohydrin to nucleic acids. Cancer Lett 23:81–90
Oesch F, Doerjer G (1982) Detection of N2,3-ethenoguanine in DNA after treatment with chloroacetaldehyde in vitro. Carcinogenesis 3:663–665
Osterman-Golkar S, Hultmark D, Segerbäck D, Calleman CJ, Göthe R, Ehrenberg L, Wachtmeister CA (1977) Alkylation of DNA and proteins in mice exposed to vinyl chloride. Biochem Biophys Res Commun 76:259–266
Ottenwälder H, Laib RJ, Bolt HM (1979) Alkylation of RNA by vinyl bromide metabolites in vitro and in vivo. Arch Toxicol 41:279–286
Ozawa N, Guengerich FP (1983) Evidence for formation of an S-[2(N-guanyl)ethyl] glytathione adduct in glutathione-mediated binding of the carcinogen 1,2-dibromoethane to DNA. Proc Natl Acad Sci USA 80:5266–5270
Parchman LG, Magee PN (1982) Metabolism of 14C-trichloroethylene to 14CO2 and interaction of a metabolite with liver DNA in rats and mice. J Toxicol Environ Health 9:797–813
Peter H, Schwarz M, Mathiasch B, Appel KE, Bolt HM (1983) A note on synthesis and reactivity towards DNA of glycidonitrile, the epoxide of acrylonitrile. Carcinogenesis 4:235–237
Peter H, Appel KE, Berg R, Bolt HM (1983a) Irreversible binding of acrylonitrile to nucleic acids. Xenobiotica 13:19–25
Peter H, Laib RJ, Ottenwälder H, Topp H, Rupprich N, Bolt HM (1985) DNA-binding assay of methyl chloride. Arch Toxicol 57:84–87
Reitz RH, Watanabe PG, McKenna MJ, Quast JF, Gehring PJ (1980) Effects of vinylidene chloride on DNA synthesis and DNA repair in the rat and mouse: a comparative study with dimethylnitrosamine. Toxicol Appl Pharmacol 52:357–370
Reitz RH, Fox TR, Ramsey JG, Quast JF, Langvardt PW, Watanabe PG (1982) Pharmacokinetics and macromolecular interactions of ethylene dichloride in rats after inhalation or gavage. Toxicol Appl Pharmacol 62:190–204
Ribovich ML, Miller JA, Miller EC, Timmins LG (1982) Labeled 1,N6-ethenoadenosine and 3,N4-ethenocytidine in hepatic RNA of mice given ethyl-1,2-3H or ethyl-1-14C ethyl carbamate (urethan). Carcinogenesis 3:539–546
Rocchi P, Prodi G, Grilli S, Ferreri AM (1973) In vivo and in vitro binding of carbon tetrachloride with nucleic acids and proteins in rat and mouse liver. Int J Cancer 11:419–425
Scherer E, Steward AP, Emmelot P (1980) Formation of precancerous islands in rat liver and modification of DNA by ethyl carbamate. In: Holmstedt B, Lauwerys R, Mercier M, Roberfroid M (eds) Mechanisms of toxicity and hazard evaluation, vol 8, Development in toxicology and environmental science. Elsevier/North-Holland, Amsterdam, pp 249–254
Scherer E, Winterwerp H, Emmelot P (1986) Modification of DNA and metabolites of ethyl carbamate in vivo: formation of 7-(2-oxoethyl)guanine and its sensitive determination by reductive tritiation using (3H)-sodium borohydride. In: Bartsch H, Singer B (eds) The role of cyclic nucleic acid adducts in carcinogenesis and mutagenesis. International Agency for Research on Cancer, Lyon
Schumann AM, Quast JF, Watanabe PG (1980) The pharmacokinetics and macromolecular interactions of perchloroethylene in mice and rats as related to oncogenicity. Toxicol Appl Pharmacol 55:207–219
Simon P, Filser JG, Bolt HM (1985) Metabolism and pharmacokinetics of vinyl acetate. Arch Toxicol 157:191–195
Simon P, Ottenwälder H, Bolt HM (1985a) Vinyl acetate: DNA-binding assay in vivo. Toxicol Lett 27:115–120
Singer B, Abbott LG, Spengler SJ (1984) Assessment of mutagenic efficiency of two carcinogen-modified nucleosides, 1,N6-ethenooxyadenosine and O4-methyldeoxythymidine, using polymerases of high fidelity. Carcinogenesis 5:1165–1171
Stoff WT, Quast JF, Watanabe PG (1982) The pharmacokinetics and macromolecular interactions of trichloroethylene in mice and rats. Toxicol Appl Pharmacol 62:137–151
Vadi HV, Schasteen CS, Reed DJ (1985) Interactions of S-(2-haloethyl)mercapturic acid and analogs with plasmid DNA. Toxicol Appl Pharmacol 80:386–396
White RD, Sipes IG, Gandolfi AJ, Bowden GT (1981) Characterization of the hepatic DNA damage caused by 1,2-dibromoethane using the alkaline elution technique. Carcinogenesis 2:839–844
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bolt, H.M., Laib, R.J., Peter, H. et al. DNA adducts of halogenated hydrocarbons. J Cancer Res Clin Oncol 112, 92–96 (1986). https://doi.org/10.1007/BF00404388
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00404388