Summary
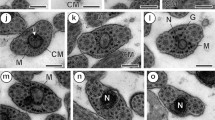
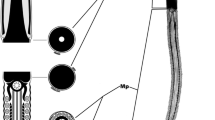
An ultrastructural comparison between the unistellate spermatozoa of the shrimps Penaeus kerathurus and P. japonicus reveals a number of similarities that are common among dendrobranchiates, but also some marked differences which would confirm the validity of a potential use of sperm structure in systematic and phylogenetic studies. Typical morphological features shared by the spermatozoa of P. kerathurus and P. japonicus are: a membrane-bound acrosomal vesicle consisting of a cap and spike; non-membrane-bound filamentous chromatin; a perinuclear cytoplasmic band including degenerative membranous organelles (mostly mitochondria), small vesicles with a dense core and parallel membrane lamellae. Discordant spermatozoal characteristics between both species involve a significantly different size (ca. 5 μm in length by ca. 3 μm in diameter in P. kerathurus; ca. 8 μm in length by ca. 4.7 μm in diameter in P. japonicus), the occurrence of intranuclear lipid droplets only in P. kerathurus and the presence of a deeper subacrosomal space in this species as compared to P. japonicus. It is very likely that the most significant difference between both species is, however, the appearance of microtubule bundles in the spermatozoon of P. japonicus. So far, the occurrence of spermatozoal microtubules in decapod crustaceans appears to be restricted to reptantian species, whereby the finding of such elements in sperm of a dendrobranchiate shrimp could be of phylogenetic interest.
Similar content being viewed by others

References
Anderson WA, Ellis RA (1967) Cytodifferentiation of the crayfish spermatozoon: acrosome formation, transformation of mitochondria and development of microtubules. Z Zellforsch 77:80–94
Brown A Jr, Talbot P, Summers RG, Clark WH Jr (1977) Comparative analysis of decapod sperm. J Cell Biol 75:170A
Burkenroad MD (1981) The higher taxonomy and evolution of Decapoda (Crustacea). Trans San Diego Soc Nat Hist 19:251–268
Clark WH Jr, Talbot P, Neal RA, Mock CR, Salser BR (1973) In vitro fertilization with non-motile spermatozoa of the brown shrimp Penaeus aztecus. Mar Biol 22:353–354
Demestre M, Fortuño J-M (1992) Reproduction of the deep-water shrimp Aristeus antennatus (Decapoda, Dendrobranchiata). Mar Ecol Prog Ser 84:41–51
Demestre M, Cortadellas N, Durfort M (1993) Ultrastructura de les espermatides de la gamba, Aristeus antennatus (Crustaci, Decapoda) (in Catalan). Biologia de la Reproducció 3:14–17
Dougherty WJ, Dougherty MM (1989) Electron microscopical and histochemical observations on melanized sperm and spermatophores of pond-cultured shrimp, Penaeus vannamei. J Invertebr Pathol 54:331–343
Dudenhausen EE, Talbot P (1982) An ultrastructural analysis of mature sperm from the crayfish Pacifastacus leniusculus Dana. Int J Invert Repord 5:149–159
Felgenhauer BL, Abele LG, Kim W (1988) Reproductive morphology of the anchialine shrimp Procaris ascensionis (Decapoda: Procarididae). J Crustac Biol 8:333–339
Felgenhauer BE, Abele LG (1991) Morphological diversity of decapod spermatozoa. In: Bauer RT, Martin JW (eds) Crustacean sexual biology. Columbia University Press, pp 322–341
Haley SR (1986) Ultrastructure of spermatogenesis in the Hawaiian red lobster, Enoplometopus occidentalis (Randall). J Morphol 190:81–92
Hinsch GW (1969) Microtubules in the sperm of the spider crab, Libinia emarginata L. J Ultrastruct Res 29:525–534
Hinsch GW (1973) Sperm structure of Oxyrhyncha. Can J Zool 51:421–426
Hinsch GW (1980) Spermiogenesis in Coenobita clypeatus, I. Sperm structure. Int J Invert Reprod 2:189–198
Hinsch GW (1986) A comparison of sperm morphologies, transfer and sperm mass storage between two species of crab, Ovalipes ocellatus and Libinia emarginata. Int J Invert Reprod Dev 10:79–87
Hinsch GW (1991) Ultrastructure of the sperm and spermatophores of the anomuran crab Pleuroncodes planipes. J Crustac Biol 11:17–22
Jamieson BGM (1989a) A comparison of the spermatozoa of Oratosquilla stephensoni and Squilla mantis (Crustacea, Stomatopoda) with comments of the phylogeny of the Malacostraca. Zool Scr 18:509–517
Jamieson BGM (1989b) Ultrastructural comparison of the spermatozoa of Ranina ranina (Oxystomata) and of other crabs exemplified by Portunus pelagicus (Brachygnatha) (Crustacea, Brachyura). Zoomorphology 109:103–111
Jamieson BGM (1989c) The ultrastructure of the spermatozoa of four species of xanthid crabs (Crustacea, Brachyura, Xanthidae). J Submicrosc Cytol Pathol 21:579–584
Jamieson BGM (1990) The ultrastructure of the spermatozoa of Petalomera lateralis (Gray) (Crustacea, Brachyura, Dromiacea) and its phylogenetic significance. Int J Invert Reprod Dev 17:39–45
Jamieson BGM (1991) Ultrastructure and phylogeny of crustacean spermatozoa. Mem Queensl Mus 31:109–142
Jamieson BGM, Tudge CC (1990) Dorippids are Heterotremata: evidence from ultrastructure of the spermatozoa of Neodorippe astuta (Dorippidae) and Portumus pelagicus (Portunidae) Brachyura: Decapoda. Mar Biol 106:347–354
Kleve MG, Yudin AI, Clark WH Jr (1980) Fine structure of the unistellate sperm of the shrimp, Sicyonia ingentis (Natantia). Tissue Cell 12:29–45
Leung-Trujillo JR, Lawrence AL (1987) Observations on the decline in sperm quality of Penaeus setiferus under laboratory conditions. Aquaculture 65:363–370
López-Camps J, Bargalló R, Bozzo MG, Durfort M, Fontarnau R (1981) The spermatogenesis of crustaceans. VII. Review of spermatozoon of the crayfish Astacus astacus (Malacostraca, Decapoda, Macrura, Reptantia). Gamete Res 4:65–82
McKnight CE, Hinsch GW (1986) Sperm maturation and ultrastructure in Scyllarus chacei. Tissue Cell 18:257–266
Medina A (1994) Spermiogenesis and sperm structure in the shrimp Parapenaeus longirostris (Crustacea, Dendrobranchiata). Comparative aspects among decapods. Mar Biol in press
Medina A, Rodríguez A (1992) Spermiogenesis and sperm structure in the crab Uca tangeri (Crustacea, Brachyura), with special reference to the acrosome differentiation. Zoomorphology 111:161–165
Ogawa Y, Kakuda S (1987) Scanning electron microscopic observations on the spermatozoa of the prawn Penaeus japonicus. Nippon Suisan Gakkaishi 53:975–977
Pochon-Masson J (1965) L'ultrastructure des épines du spermatozoïde chez les Décapodes (Macroures, Anomoures, Brachyoures). CR Acad Sci Paris 260:3762–3764
Pochon-Masson J (1968a) L'ultrastructure des spermatozoïdes vésiculaires chez les crustacés décapodes avant et au cours de leur dévagination expérimentale. I. Brachyoures et Anomoures. Ann Sci Nat Zool Paris 10:1–100
Pochon-Masson J (1968b) L'ultrastructure des spermatozoïdes vésiculaires chez les crustacés décapodes avant et au cours de leur dévagination expérimentale. II. Macroures. Discussion et conclusions. Ann Sci Nat Zool Paris 10:367–454
Ro S, Talbot P, Leung-Trujillo J, Lawrence AL (1990) Structure and function of the vas deferens in the shrimp Penaeus setiferus: Segments 1–3. J Crustac Biol 10:455–468
Rodríguez A (1985) Biología del langostino Penaeus kerathurus (Forskal 1775) del golfo de Cádiz. I. Reproducción. Inv Pesq 49:581–595
Shigekawa K, Clark WH Jr (1968) Spermiogenesis in the marine shrimp, Sicyonia ingentis. Dev Growth Differ 28:5–112
Spurr AR (1969) A low viscosity epoxy-resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Talbot P, Summers RG (1978) The structure of sperm from Panulirus, the spiny lobster, with special regard to the acrosome. J Ultrastruct Res 64:341–351
Talbot P, Chanmanon P (1980) The structure of sperm from the lobster, Homarus americanus. J Ultrastruct Res 70:275–286
Talbot P, Howard D, Leung-Trujillo J, Lee TW, Li W-Y, Ro H, Lawrence AL (1989) Characterization of male reproductive tract degenerative syndrome in captive penaeid shrimp (Penaeus setiferus). Aquaculture 78:365–377
Tudge CC (1992) Comparative ultrastructure of hermit crab spermatozoa (Decapoda: Anomura: Paguroidea). J Crustac Biol 12:397–409
Tudge CC, Jamieson BGM (1991) Ultrastructure of the mature spermatozoon of the coconut crab Birgus latro (Coenobitidae: Paguroidea: Decapoda). Mar Biol 108:395–402
Yasuzumi G, Lee KJ (1966) Spermatogenesis in animals as revealed by electron microscopy. XVI. The microtubular structure and sites of thiamine pyrophosphatase activity in premature sperm of the japanese crayfish. Z Zellforsch 73:384–404
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Medina, A., Mourente, G., de la Rosa, I.L. et al. Spermatozoal ultrastructure of Penaeus kerathurus and Penaeus japonicus (Crustacea, Dendrobranchiata). Zoomorphology 114, 161–167 (1994). https://doi.org/10.1007/BF00403263
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00403263