Abstract
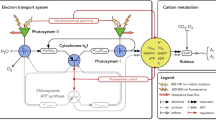
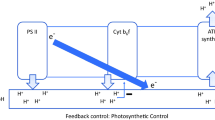
In order to obtain information on fractional control of photosynthesis by individual catalysts, catalytic activities in photosynthetic electron transport and carbon metabolism were modified by the addition of inhibitors, and the effect on photosynthetic flux was measured using chloroplasts of Spinacia oleracea L. In thylakoids with coupled electron transport, light-limited electron flow to ferricyanide was largely controlled by the QB protein of the electron-transport chain. Fractional control by the cytochrome f/b 6 complex was insignificant under these conditions. Control by the cytochrome f/b 6 complex dominated at high energy fluence rates where the contribution to control of the QB protein was very small. Uncoupling shifted control from the cytochrome f/b 6 complex to the QB protein. Control of electron flow was more complex in assimilating chloroplasts than in thylakoids. The contributions of the cytochrome f/b 6 complex and of the QB protein to control were smaller in intact chloroplasts than in thylakoids. Thus, even though the transit time for an electron through the electron-transport chain may be below 5 ms in leaves, oxidation of plastohydroquinone was only partially responsible for limiting photosynthesis under conditions of light and CO2 saturation. The energy fluence rate influenced control coefficients. Fractional control of photosynthesis by the ATP synthetase, the cytochrome f/b 6 complex and by ribulose-1,5-bisphosphate carboxylase increased with increasing fluence rates, whereas the contributions of the QB protein and of enzymes sensitive to SH-blocking agents decreased. The results show that the burdens of control are borne by several components of the photosynthetic apparatus, and that burdens are shifted as conditions for photosynthesis change.
Similar content being viewed by others
Abbreviations
- Chl:
-
chlorophyll
- DCMU:
-
3-(3′,4′-dichlorophenyl)-1,1-dimethylurea
- DNP-INT:
-
2,4-dinitro phenylether of 2-iodo-4-nitrothymol
- pCMBS:
-
p-chloromercuribenzosulfonate
References
Bendall, D.S. (1982) Photosynthetic cytochromes of oxygenic organisms. Biochim. Biophys. Acta 683, 119–151
Buchanan, B.B. (1980) Role of light in the regulation of chloroplast enzymes. Annu. Rev. Plant Physiol. 31, 341–374
Dietz, K.-J. (1986) An evaluation of light and CO2 limitation of leaf photosynthesis by CO2 gas exchange analysis. Planta 167, 260–263
Dietz, K.-J., Foyer, C. (1986) The relationship between phosphate status and photosynthesis in leaves. Reversibility of the effects of phosphate deficiency on photosynthesis. Planta 167, 376–381
Dietz, K.-J., Heber, U. (1984) Rate-limiting factors in leaf photosynthesis. I. Carbon fluxes in the Calvin cycle. Biochim. Biophys. Acta 767, 432–443
Dietz, K.-J., Neimanis, S., Heber, U. (1984) Rate-limiting factors in leaf photosynthesis. II. Electron transport. Biochim. Biophys. Acta 767, 444–450
Dietz, K.-J., Schreiber, U., Heber, U. (1985) The relationship between the redox state of QA and photosynthesis in leaves at various carbon dioxide, oxygen and light regimes. Planta 166, 219–226
Good, N.E., Izawa, S. (1973) Inhibition of photosynthesis. In: Metabolic inhibitors, vol. IV, pp 179–214, Hochster, R.M., Quastel, J.H., eds. Academic Press, New York
Hachnel, W. (1984) Photosynthetic electron transport in higher plants. Annu. Rev. Plant Physiol. 35, 659–693
Hauska, G., Hurt, E., Gabellini, N., Lockau, W. (1983) Comparative aspects of quinol-cytochrome c/plastocyanin oxidoreductases. Biochim. Biophys. Acta 726, 97–133
Heber, U., Boardman, N.K., Anderson, J.M. (1976a) Cytochrome b 563 redox changes in intact CO2-fixing spinach chloroplasts and in developing pea chloroplasts. Biochim. Biophys Acta 423, 275–292
Heber, U., Boardman, N.K., Anderson, J.M. (1976b) Effects of pH and oxygen on photosynthetic reactions of intact chloroplasts. Plant Physiol. 57, 277–283
Heber, U., Neimanis, S., Dietz, K.-J., Viil, J. (1986) Assimilatory power as a driving force in photosynthesis. Biochim. Biophys. Acta 852, 144–155
Heber, U., Santarius, K.A. (1970) Direct and indirect transport of ATP and ADP across the chloroplast envelope. Z. Naturforsch. 25b, 718–728
Hill, R., Hartree, E.F. (1953) Hematin compounds in plants. Annu. Rev. Plant Physiol. 4, 115–150
Izawa, S., Good, N.E. (1965) The number of sites sensitive to 3-(3,4-dichlorophenyl)-1,1-dimethylurea, 3-(4-chlorophenyl)-1,1-dimethylurea and 2-chloro-4-(2-propylamino)-6-ethylamino-s-triazine in isolated chloroplasts. Biochim. Biophys. Acta 102, 20–38
Izawa, S., Winget, G.D., Good, N.E. (1966) Phlorizin, a specific inhibitor of photophosphorylation and phosphorylationcoupled electron transport in chloroplasts. Biochem. Biophys. Res. Comm. 22, 223–226
Jensen, R.G., Bassham, J.A. (1966) Photosynthesis by isolated chloroplasts. Proc. Natl. Acad. Sci. USA 56, 1095–1101
Kacser, H., Burns, J.A. (1979) Molecular democracy: Who shares the controls? Biochem. Soc. Trans. 7, 1149–1160
Laasch, H., Pfister, K., Urbach, W. (1982) High- and lowaffinity binding of photosystem II-herbicides to isolated thylakoid membranes and intact algal cells. Z. Naturforsch. 37c, 620–631
Laisk, A., Walker, D.A. (1986) Control of phosphate turnover as a rate-limiting factor and possible cause of oscillations in photosynthesis: A mathematical model. Proc. R. Soc. London, Ser. B 227, 281–302
Leegood, R.C., Kobayshi, Y., Neimanis, S., Walker, D.A., Heber, U. (1982) Cooperative activation of chloroplast fructose-1,6-bisphosphatase by reductant, pH and substrate. Biochim. Biophys. Acta 682, 168–178
Li, Y.S., Gibbs, M. (1985) Pyridoxal phosphate and 3-phosphoglycerate overcome the D,l-glyceraldehyde inhibition of carbon dioxide fixation by intact chloroplasts. Plant Physiol. 77, 42
Madsen, N.B. (1963) Mercaptide forming agents. In Metabolic inhibitors, vol 2, pp. 119–143, Hochster, R.M., Quastel, J.H., eds. Academic Press, New York
McCarty, R.E. (1980) Deineation of the mechanism of ATP synthesis in chloroplasts: use of uncoplers, energy transfer inhibitors, and modifiers of coupling factor I. Methods Enzymol. 69, 719–728
Ohmori, M., Gimmler, H., Schreiber, U., Heber, U. (1985) Relative insensitivity of photosynthesis to the dissipation of a transthylakoid proton gradient in intact chloroplasts. Physiol. Vég. 23, 801–812
Sharkey, T.D., Stitt, M., Heinecke, D., Gerhardt, R., Raschke, K., Heldt, H.W. (1986) Limitation of photosynthesis by carbon metabolism. II. Oxygen insensitive CO2 uptake results from limitation of triosephosphate utilization. Plant Physiol. 81, 1123–1129
Sivak, M.N., Walker, D.A. (1986) Photosynthesis in vivo can be limited by phosphate supply. New Phytol. 102, 499–512
Slabas, A.R., Walker, D.A. (1978) Inhibition of spinach phosphoribulokinase by D,l-glyceraldehyde. Biochem. J. 153, 613–619
Stitt, M. (1986) Limitation of photosynthesis by carbon metabolism. I. Evidence for excess electron transport capacity in leaves carrying out photosynthesis in saturating light and CO2. Plant Physiol. 81, 1115–1122
Stitt, M. (1987) Limitation of photosynthesis by sucrose synthesis. In: Progress in photosynthesis research, vol. 3, pp. 685–692, Biggins, J., ed. Martinus Nijhoff Publishers, Dordrecht Boston Lancaster
Takahama, U., Shimidzu-Takahama, M., Heber, U. (1981) The redox state of the NADP system in illuminated chloroplasts. Biochim. Biophys. Acta 637, 530–539
Trebst, A. (1980) Inhibitors in electron flow: Tools for the functional and structural localization of carriers and energy conservation sites. Methods Enzymol. 69, 675–715
Trebst, A., Wietoska, H., Draber, W., Knops, H.J. (1978) The inhibition of photosynthetic electron flow in chloroplasts by the dinitrophenylether of bromo-or iodo-nitrothymol. Z. Naturforsch. 33c, 919–927
von Caemmerer, S., Farquhar, G.D. (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387
Witt, H.T. (1979) Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. Biochim. Biophys. Acta 505, 355–427
Wishnick, M., Lane, M.D. (1971) Ribulose diphosphate carboxylase from spinach leaves. Methods Enzymol. 23, 570–577
Woodrow, I.E. (1986) Control of the rate of photosynthetic carbon dioxide fixation. Biochim. Biophys. Acta 851, 181–192
Woodrow, I.E., Ball, J.T., Berry, J.A. (1986) A general expression for the control of the rate of photosynthetic CO2 fixation by stomata, the boundary layer and radiation exchange. In: Progress in photosynthesis research, vol. 4, pp. 225–228, Biggins, J. ed. Martinus Nijhoff Publishers, Dordrecht Boston Lancaster
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Heber, U., Neimanis, S. & Dietz, K.J. Fractional control of photosynthesis by the QB protein, the cytochrome f/b 6 complex and other components of the photosynthesic apparatus. Planta 173, 267–274 (1988). https://doi.org/10.1007/BF00403020
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00403020