Abstract
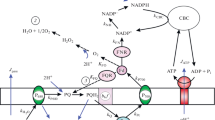
A computer model is used to analyze fluxes of SO2 from polluted air into leaves and the intracellular distribution of sulfur species derived from SO2. The analysis considers only effects of acidification and of anion accumulation. (i) The SO2 flux into leaves is practically exclusively controlled by the boundary-layer resistance of leaves to gas diffusion and by stomatal opening. At constant stomatal opening, flux is proportional to the concentration of SO2 in air. (ii) The sink capacity of cellular compartments for SO2 depends on intracellular pH and the intracellular localization of reactions capable of oxidizing or reducing SO2. In the mesophyll of illuminated leaves, the chloroplasts possess the highest trapping potential for SO2. (iii) If intracellular ion transport were insignificant, and if bisulfite and sulfite could not be oxidized or reduced, leaves with opened stomata would rapidly be killed both by the accumulation of sulfites and by acidification of chloroplasts and cytosol even if SO2 levels in air did not exceed concentrations thought to be permissible. Acidification and sulfite accumulation would remain confined largely to the chloroplasts and to the cytosol under these conditions. (iv) Transport of bisulfite and protons produced by hydration of SO2 into the vacuole cannot solve the problem of cytoplasmic accumulation of bisulfite and sulfite and of cytoplasmic acidification, because SO2 generated in the acidic vacuole from the bisulfite anion would diffuse back into the cytoplasm. (v) Oxidation to sulfate which is known to occur mainly in the chloroplasts can solve the problem of cytoplasmic sulfite and bisulfite accumulation, but aggravates the problem of chloroplastic and cytosolic acidification. (vi) A temporary solution to the problem of acidification requires the transfer of H+ and sulfate into the vacuole. This transport needs to be energized. The storage capacity of the vacuole for protons and sulfate defines the extent to which SO2 can be detoxified by oxidation and removal of the resulting protons and sulfate anions from the cytoplasm. Calculations show that even at atmospheric levels of SO2 thought to be tolerable, known vacuolar buffer capacities are insufficient to cope with proton production during oxidation of SO2 to sulfate within a vegetation period. (vii) A permanent solution to the problem of acidification is the removal of protons. Protons are consumed during the reduction of sulfate to sulfide. Proteins and peptides contain sulfur at the level of sulfide. During photosynthesis in the presence of the permissible concentration of 0.05μl·l-1 SO2, sulfur may be deposited in plants at a ratio not far from 1/500 in relation to carbon. The content of reduced sulfur to carbon is similar to that ratio only in fast-growing, protein-rich plants. Such plants may experience little difficulty in detoxifying SO2. In contrast, many trees may contain reduced sulfur at a ratio as low as 1/10 000 in relation to carbon. Excess sulfur deposited in such trees during photosynthesis in polluted air gives rise to sulfate and protons. If detoxification of SO2 by reduction is inadequate, and if the storage capacity of the vacuoles for protons and sulfate is exhausted, damage is unavoidable. Calculations indicate that trees with a low ratio of reduced S to C cannot tolerate long-term exposure to concentrations of SO2 as low as 0.02 or 0.03 μl·l-1 which so far have been considered to be non-toxic to sensitive plant species.
Similar content being viewed by others
References
Asada, K., Kiso, K. (1973) Initiation of aerobic oxidation of sulfite by illuminated spinach chloroplasts. Eur. J Biochem. 33, 253–257
Baldry, C.W., Cockburn, W., Walker, D.A. (1968) Inhibition by sulfate of the oxygen evolution associated with photosynthetic carbon assimiliation. Biochim. Biophys. Acta 18, 476–483
Beilke, S., Gravenhorst, G. (1978) Heterogeneous SO2-oxidation in the droplet phase. Atmos. Environ. 12, 231–239
Beyschlag, W., Wedler, M., Lange, O.L., Heber, U. (1987) Einfluß einer Magnesiumdüngung auf Photosynthese und Transpiration von Fichten an einem Magnesium-Mangelstandort im Fichtelgebirge. Allg. Forstzeitschrift 27/28/29, 738–741
Brimblecombe, P., Spedding, D.J. (1974) The catalytic oxidation of micromolar aqueous sulphur dioxide. I. Oxidation in dilute solutions containing iron (III). Atmosph. Environ. 8, 937–945
Buchanan, B.B. (1980) Role of light in the regulation of chloroplast enzymes. Annu. Rev. Plant Physiol. 31, 341–374
Dijkshoorn, W., van Wijk, A.L. (1967) The sulphur requirements of plants as evidenced by the sulphur-nitrogen ratio in the organic matter. Plant Soil 26, 129–157
Faller, N., Herwig, K., Kühn, H. (1970) Die Aufnahme von Schwefeldioxyd (S35O2) aus der Luft. II. Aufnahme, Umbau und Verteilung in der Pflanze. Plant Soil 33, 283–295
Garnier, R.V., Latzko, E. (1972) Regulation of photosynthetic C-1-fructose-diphosphatase. In: Proc. 2nd Int. Congr. on Photosynthesis Research, pp. 1839–1845, Forti, D., Avron, M., Melandri, A., eds. W. Junk, The Hague
Garsed, S.G. (1985) SO2-uptake and transport. In: Sulfur dioxide and vegetation. Physiology, ecology and policy issues, pp. 75–95, Winner, W.E., Mooney, H.A., Goldstein, R.A., eds. Stanford University Press, Stanford, Cal., USA
Garsed, S.G., Read, D.J. (1977a) Sulphur dioxide metabolism in soy-bean, Glycine max, var. biloxi. I. The effects of light and dark on the uptake and translocation of 35SO2. New Phytol. 78, 111–119
Garsed, S.G., Read, D.J. (1977b) Sulphur dioxide metabolism in soy-bean, Glycine max var biloxi. II. Biochemical distribution of 35SO2 roducts. New Phytol. 99, 583–592
Grill, D., Esterbauer, H., Scharner, M., Felgitsch, C.H. (1980) Effect of sulfur dioxide on protein-SH in needles of Picea abies. Eur. J. Forest Pathol. 10, 263–267
Guderian, R. (1970) Untersuchungen über quantitative Beziehungen zwischen dem Schwefelgehalt von Pflanzen und dem Schwefelgehalt der Luft. Z. Pflanzenkrank. 77, 200–220
Guderian, R. (1977) Air pollution. Phytotoxicity of acidic gases and its significance in air pollution control. Springer, Berlin
Hällgren, J.E. (1978) Physiological and biochemical effects of sulfur dioxide on plants. In: Sulfur in the environment. Part II. Ecological impacts, pp. 163–209, Nriagu, J.O., ed. Wiley New York
Hällgren, J.-E., Fredriksson, S.-A. (1982) Emission of sulfide from sulfur dioxide-fumigated pine trees. Plant Physiol. 70, 456–459
Hampp, R., Spedding, D.J., Ziegler, I. Ziegler, H. (1980) The efflux of inorganic sulphur from spinach chloroplasts. Z. Pflanzenphysiol. 99, 113–119
Hampp, R., Ziegler, I. (1977) Sulfate and sulfite translocation via the phosphate translocator of the inner envelope membrane of chloroplasts. Planta 137, 309–312
Heber, U., Heldt, H.-W., (1981) The chloroplast envelope: structure, function, and role in leaf metabolism. Annu. Rev. Plant Physiol. 32, 139–168
Heber, U., Laisk, A., Pfanz, H., Lange, O.L. (1987) Wann ist SO2 Nähr-und wann Schadstoff? Ein Beitrag zum Waldsterbensproblem. Allg. Forstzeitschrift 27/28/29, 700–705
Hocking, A., Hocking, M.B. (1977) Equilibrium solubility of trace atmospheric sulphur dioxide in water and its bearing on air pollution injury to plants. Environ. Pollut. 13, 57–64
Laisk, A., Pfanz, H., Schramm, M.J., Heber, U. (1987) SO2 fluxes into different cellular compartments of leaves photosynthesizing in a polluted atmosphere. I. Computer analysis. Planta 173, 230–240
Leegood, R.C., Kobayashi, Y., Neimanis, S., Walker, D.A., Heber, U. (1982) Co-operative activation of chloroplast fructose-1,6-bisphosphatase by reductant, pH and substrate. Biochim. Biophys. Acta 682, 168–178
Lyr, H., Polster, H., Fiedler, H.-J. (1967) Gehölzphysiologie. Gustav Fischer, Jena
Martinoia, E., Schramm, M.J., Kaiser, G., Kaiser, W.M., Heber, U. (1986) Transport of anions in isolated barley vacuoles. I. Permeability to anions and evidence for a Cl- uptake system. Plant Physiol. 80, 895–901
Mourioux, G., Douce, R. (1979) Transport du sulfate à travers la double membrane limitante ou l'envelope, des chloroplasts d'epinard. Biochimie 61, 1283–1292
Oja, V., Laisk, A., Heber, U. (1986) Light induced alkalization of the chloroplast stroma in vivo as estimated from the CO2 capacity of intact sunflower leaves. Biochim. Biophys. Acta 849, 355–365
Olszyk, D.M., Tingey, D.T. (1985) Interspecific variation in SO2-flux. Leaf surface versus internal flux, and components of leaf conductance. Plant Physiol. 79, 949–956
Pfanz, H., Heber, U. (1985) Protonenflüsse und zelluläre Pufferkapazitäten in Blättern bei SO2-Belastung. In PBWU ed. Proc. of the International Workshop on Physiology and Biochemistry of Stressed Plants. GSF-Bericht 44/85, Neuherberg, pp. 103–113
Pfanz, H., Heber, U. (1986) Buffer capacities of leaves, leaf cells, and leaf cell organelles in relation to fluxes of potentially acidic gases. Plant Physiol. 81, 597–602
Pfanz, H., Martinoia, E., Lange O.L., Heber, U. (1987a) Mesophyll resistances to SO2 fluxes into leaves. Plant Physiol., in press
Pfanz, H., Martinoia, E., Lange, O.L., Heber, U. (1987b) Fluxes of SO2 into leaf cells and cellular acidification by SO2. Plant Physiol., in press
Raven, J.A., Smith, F.A. (1981) Cytoplasmic pH regulation and electrogenic H+ extrusion. In: Commentaries in plant science, vol 2. pp. 27–39, Smith, H., ed. Pergamon Press, Elmsford New York
Rennenberg, H. (1984) The fate of excess sulfur in higher plants. Annu. Rev. Plant Physiol. 35, 121–153
Rennenberg, H., Filner, P. (1982) Stimulation of H2S-emission from pumpkin leaves by inhibition of glutathione synthesis. Plant Physiol. 69, 766–770
Rennenberg, H., Filner, P. (1983) Developmental changes in the potential for H2S emission in cucurbit plants. Plant Physiol. 71, 269–275
Salisbury, F.B., Ross, C.W. (1978) Plant physiology. Wadsworth, Belmont
Schulze, E.-D. (1970) Der Gaswechsel der Buche (Fagus silvatica L.) in Abhängigkeit von den Klimafaktoren im Freiland. Flora 159, 177–232
Schulze, E.-D. (1981) Carbon gain and wood production in trees of deciduous beech (Fagus silvatica) and trees of evergreen spruce (Picea excelsa). Mitt. Forstl. Bundesversuchsanstalt Wien 142, 102–123
Schulze, E.-D. (1982) Plant life forms and their carbon, water and nutrient relations. In: Encyclopedia of plant physiology, N.S., vol. 12B: Physiological plant ecology, II, pp. 615–676, Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H., eds. Springer, Berlin Heidelberg New York
Sekjya, J., Wilson, L.G., Filner, P. (1982) Resistance to injury by sulfur dioxide. Correlation with its reduction to, and emission of, hydrogen sulfide in Cucurbitaceae. Plant Physiol. 70, 437–441
Weigl, J., Ziegler, H. (1962) Die räumliche Verteilung und die Art markierter Verbindungen in Spinatblättern nach Begasung mit 35SO2. Planta 58, 435–447
Wilson, L.G., Bressan, R.A., Filner, P. (1978) Light-dependent emission of hydrogen sulfide from plants. Plant Physiol. 61, 184–189
Ziegler, I., Hampp, R. (1977) Control of 35SO 2-4 and 35SO 2-3 incorporation into spinach chloroplasts during photosynthetic CO2 fixation. Planta 137, 303–307
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Laisk, A., Pfanz, H. & Heber, U. Sulfur-dioxide fluxes into different cellular compartments of leaves photosynthesizing in a polluted atmosphere. Planta 173, 241–252 (1988). https://doi.org/10.1007/BF00403016
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00403016