Summary
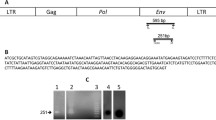
Restriction enzyme analysis and molecular hybridization assay of DNA isolated from C57B1/10 mammary adenocarcinomas induced by a combination of dimethylbenzanthracene, oestrogen, and prolactin revealed the presence of four extra copies of endogenous mouse mammary tumour virus (MMTV). PstI restriction pattern of the amplified proviral sequences indicated their identity with the proviral Unit II of endogenous MMTV. The amplified proviruses are hypomethylated and expressed in a hormone-dependent fashion. Their internal structure is slightly modified, since an additional EcoRI recognition site is present within the proviral genomic DNA. Selective amplification of Unit II MMTV provirus in the course of mammary tumourigenesis initiated by chemical carcinogens and hormones is compatible with the accepted multifactorial nature of this process, and is interpreted in terms of the insertional mutagenesis model for MMTV-induced oncogenesis. However, sequences of cellular DNA, adjacent to the amplified Unit II proviruses, show no homology to the integration domains int-1 and int-2 common to exogenous MMTV.
Similar content being viewed by others
References
Altaner C, Zajac V, Ban J (1982) A simple and inexpensive method for detection of BLV infected cattle based on a modified ELISA principle. Zbl Vet Med 29:583–590
Breznik T, Choen JC (1982) Altered methylation of endogenous viral promoter sequences during mammary carcinogenesis. Nature London 295:255–257
Butel JS, Dusing-Schwarzt S, Socher H, Medina D (1981) Partial expression of endogenous mouse mammary tumor virus in mammary tumous induced in Balb/c mice by chemical, hormonal, and physical agents. J Virol 38:571–580
Cohen JC, Majors JE, Varmus HE (1979a) Organization of mouse mammary tumor virus-specific DNA endogenous to Balb/c mice. J Virol 32:483–494
Cohen JC, Shank PR, Morris IL, Cardiff R, Varmus HE (1979b) Integration of the DNA of mouse mammary tumor virus-infected normal and neoplastic tissue of the mouse. Cell 16:333–345
Cohen JC, Varmus HE (1980) Proviruses of mouse mammary tumor virus in normal and neoplastic tissues from GR and C3Hf mouse strains. J Virol 35:298–305
Dudley JP, Butel JS, Socher SH, Rosen JM (1978) Detection of mouse mammary tumor virus RNA in Balb/c tumor cell lines of non-viral etiologies. J. Virol 28:743–752
Etkind PR, Sarkar NH (1983) Integration of new endogenous mouse mammary tumor virus proviral DNA at common sites in the DNA of mammary tumors of C3Hf mice and hypomethylation of the endogenous mouse mammary tumor virus proviral DNA in C3Hf mammary tumors and spleens. J Virol 45:114–123
Fanning TG, Vassos AB, Cardiff RD (1982) Methylation and amplification of mouse mammary tumor virus DNA in normal, premalignant, and malignant cells of GR-A mice. J Virol 41:1007–1013
Fasel N, Pearson K, Buetti E, Diggelmann H (1982) The region of mouse mammary tumor virus, lacking 516 nucleotides of the 5′ long terminal repeat sequence, can be expressed in a hormone-responsive fashion. J Gen Virol 64:567–577
Fine DL, Plowman JK, Kelly SP, Authur LO, Hillman EA (1974) Enhanced production of mouse mammary tumor virus in dexamethasone-treated 5-iododeoxyuridine-stimulated mammary tumor cells in culture. J Natl Cancer Inst 52:1881–1886
Fung YT, Lewis WG, Crittenden LB, Kung HJ (1983) Activation of the cellular oncogene c-erb by LTR insertion: Molecular basis for induction of erythroblastosis by avian leukosis virus. Cell 33:357–368
Graham DE, Medina D, Smith GH (1984) Increased concentration of an indigenous proviral mouse mammary tumor virus long terminal repeat-containing transcript is associated with neoplastic transformation of mammary epithelium in C3H/Sm mice. J Virol 49:819–827
Groner B, Buetti E, Diggelmann H, Hynes NE (1980) Characterization of endogenous and exogenous mouse mammary tumor virus proviral DNA with size-specific molecular clones. J Virol 36:734–745
Groner E, Hynes NE, Rahmsdorf N, Ponta H (1983) Transcription initiation of transfected mouse mammary tumor virus LTR DNA is regulated by glucocorticoid hormones. Nucleic Acids Res 11:4713–4725
Hayward WS, Nell BC, Astrin SM (1981) Activation of a cellular oncogene by promoter insertion in ALV-induced lymphoid leukosis. Nature London 210:475–480
Hilgers J, Bentvelzen P (1979) Interaction between viral and genetic factors in murine mammary cancer. Adv Cancer Res 26:143–195
Jaenish R (1983) Endogenous retroviruses Cell 32:5–6
Lane MA, Sainten A, Cooper CM (1981) Activation of related transforming genes in mouse and human mammary carcinomas. Proc Natl Acad Sci USA 78:5185–5189
Long CA, Dumaswala UJ, Tancin SL, Vaidya AB (1980) Organization and expression of endogenous murine mammary tumor virus genes in mice congenic at the H-2 complex. Virology 103:167–177
Michalides R, Schlom J (1975) Relationship in nucleic acid sequences between mouse mammary tumor virus variants. Proc Natl Acad Sci USA 72:4635–4639
Michalides R, Schlom J, Dahlberg J, Perk K (1975) Biochemical properties of the bromodeoxyuridine-induced guinea pig virus. J Virol 16:1039–1046
Michalides R, Van Deenter L, Nusse R, Ropcke G, Boot L (1978) Involvement of mouse mammary tumor virus in spontaneous and hormone-induced mammary tumors in low-mammary-tumor mouse strains. J Virol 27:551–559
Michalides R, Van Deenter L, Nusse R, Nageman P (1979) Induction of mouse mammary tumor virus RNA in mammary tumors of Balb/c mouse treated with urethane, X-irradiation, and hormones. J Virol 31:63–72
Michalides R, Van Nie R, Nusse R, Hynes NE, Groner B (1981b) Mammary tumor induction loci in GR and DBAf mice contain one provirus of the mouse mammary tumor virus. Cell 23:165–173
Michalides R, Wagenaar E, Hynes NE (1981a) Mammary tumor virus proviral DNA in normal murine tissue and non-virally induced mammary tumors. J. Virol 39:367–376
McGrath CM, Prass WA, Maloney TM, Jones RF (1981) Induction of endogenous mammary tumor virus expression during hormonal induction of mammary adenocarcinomas and carcinomas of Balb/c female mice. J Natl Cancer Inst 67:841–851
Morris VL, Medeiros E, Ringold GM, Bishop JM, Varmus HE (1977) Comparison of mouse mammary tumor virus-specific DNA in inbred, wild, and Asian mice, and in tumors and normal organs from inbred mice. J Mol Biol 114:73–92
Morris VL, Vlasschaert JE, Beard CL, Milazzo MF, Bradburry WC (1980) Mammary tumors from Balb/c mice with a reported high mammary tumor incidence have acquired new mammary tumor virus DNA. Virology 100:101–109
Nusse R, Michalides R, Ropcke G, Boot LM (1980) Quantification of mouse mammary tumor virus structural proteins in hormone-induced mammary tumors of low-mammary-tumor mouse strains. Int J Cancer 25:377–383
Nusse R, Varmus HE (1982) Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31:99–109
Peters G, Brookes S, Smith R, Dickson C (1983) Tumorigenesis by mouse mammary tumor virus: Evidence for a common region for provirus integration in mammary tumors. Cell 33:369–377
Ponta H, Kennedy N, Herrlich P, Hynes NE, Groner B (1983) A deletion mutant of mouse mammary tumor virus, lacking 516 nucleotides of the 5'long terminal repeat sequence can be expressed in a hormone-responsive fashion. J Gen Virol 64:567–577
Ringold GJ, Yamamoto KR, Tomkins GM, Bishop JM, Marmus HE (1975) Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell 6:299–305
Schlom J, Michalides R, Kufe D, Hehlman R, Spiegelman S, Bentvelzen P, Hageman P (1973) A comparative study on the biologic and molecular basis of murine mammary carcinoma: a model for human breast cancer. J Natl Cancer Inst 51:541–551
Shank PR, Cohen JC, Varmus HE, Yamamoto KR, Ringold GM (1978) Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci USA 75:2112–2116
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 38:503–517
Svec J, Hlavayova E, Matoska J, Thurzo V (1979) Conditions for hormone-stimulated expression of endogenous C57B1 strain-associated mammary tumor virus genome. Neoplasma 26:539–550
Svec J, Svec P, Halcak L, Thurzo V (1982) Role of natural prostaglandins in the control of murine mammary tumor expression. J Cancer Res Exp Oncol 103:55–67
Svec J, Michalides R (1983) Acquisition of new hormone-responsive proviral sequences of mouse mammary tumor virus in cell clones derived from a C57B1/10 mammary adenocarcinoma induced by non-viral carcinogens. In: Grofova M (ed) Proceedings of the European Symposium on Oncovirology Castle of Smolenice, Czechoslovakia 1983, 1983 p 41
Taylor JM, Ilimensee R, Summers J (1976) Efficient transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta 442:324–332
Teich N, Wyke JA, Mak T, Bernstein A, Hardy W (1982) Pathogenesis of retrovirus-induced disease. In: RA Weiss, N Teich, HE Warmus, JM Coffin (eds) The molecular biology of tumor viruses, part III RNA tumor viruses Cold Spring Harbor, New York, pp 123–186
Timmermans A, Bentvelzen P, Hageman P, Calafat J (1968) Activation of mammary tumor virus in 020 strain of mice by X-irradiation and urethane. J Gen Virol 4:619–621
Traina VL, Taylor BA, Cohen CJ (1981) Genetic mapping of endogenous mouse mammary tumor viruses: Locus characterization, segregation, and chromosomal distribution. J Virol 40:735–744
Vaidya AB, Taraschi NE; Tancin SL, Long SA (1983) Regulation of endogenous murine mammary tumor virus expression in C57B1 mouse lactating mammary gland: Transcription of functional mRNA with block at the translational level. J Virol 46:818–828
Varmus HE, Quintreu N, Medeiros E, Bishop JM, Nowinski RC, Sarkar NH (1973) Transcription of mouse mammary tumor virus genes in tissues from high and low incidence mouse strains. J Mol Biol 79:663–679
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Svec, J. Proviral Unit II of endogenous mouse mammary tumour virus is selectively amplified and expressed in C57B1/10 mammary tumours induced by non-viral carcinogens. J Cancer Res Clin Oncol 110, 25–34 (1985). https://doi.org/10.1007/BF00402498
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00402498