Abstract
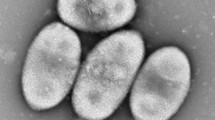
Indole (1.5 mmol/l) added to suflate-rich marine mud or sulfate-free sewage digestor sludge was anaerobically degraded within one week. Enrichments from sludge samples in defined indole-containing media with or without sulfate were selective for sulfate-reducing bacteria or mixed methanogenic associations, respectively. Other enrichments of sulfate-reducing bacteria were obtained with skatole, indoleacetate, indolepropionate, quinoline, and pyridine. From a marine enrichment with indole as sole electron donor and carbon source, an oval to rod-shaped, Gram-negative, nonsporing sulfate-reducing bacterium (strain In04) was isolated. Growth occurred in defined bicarbonate-buffered, sulfide-reduced media supplemented with vitamin B12. Furthen aromatic compounds utilized as electron donors and carbon sources were anthranilic acid and quinoline. Nonaromatic compounds used as substrates were formate, acetate, propionate, ethanol, propanol, butanol, pyruvate, malate, fumarate, and succinate. However, growth with substrates other than indole was rather slow. Thiosulfate served as an alternative electron acceptor. Complete oxidation of indole to CO2 was shown by stoichiometric measurements in batch culture with sulfate as electron acceptor. An average growth yield of 31.3 g cell dry weight was obtained per mol of indole oxidized. Pigment analysis revealed that cytochromes and menaquinone MK-7 (H2) were present. Desulfoviridin could not be detected. Strain In04 is described as new species of the new genus Desulfobacterium indolicum.
Similar content being viewed by others
References
Balba MT, Evans WC (1980) Methanogenic fermentation of naturally occurringaromatic amino acids by a microbial consortium. Biochem Soc Trans 8:625–627
Barker HA (1981) Amino acid degradation by anaerobic bacteria. Ann Rev Biochem 50:23–40
Braun K, Gibson DT (1984) Anaerobic degradation of anthranilic acid by denitrifying bacteria. Appl Environ Microbiol 48:102–107
Carlson JR, Yokoyama MT, Dickinson EO (1972) Induction of pulmonary edema and emphysema in cattle and goats with 3-methylindole. Science 176:298–299
Chmielowski J, Grossman A, Labuzek S (1965) Biochemical degradation of some phenolies during methane fermentation. Zesz Nauk Politech Seria: Inz Sanit 8:97–123
Cline E (1969) Spectrophotometric determination of hydrogensulfide in natural waters. Limnol Oceanogr 14:454–458
Collins MD, Widdel F (1986) Respiratory quinones of sulphate-reducing and sulphur-reducing bacteria: a systematic investigation. J Gen Microbiol (in press)
Elsden SR, Hilton MG, Waller JM (1976) The endproducts of the metabolism of aromatic amino acids by clostridia. Arch Microbiol 107:283–288
Evans WC (1977) Biochemistry of the bacterial catabolism of aromatic compounds in anaerobic environments. Nature 270:17–22
Fedorak PM, Hrudey SE (1984) The effects of phenole and some alkyl-phenolics on batch anaerobic methanogenesis. Water Res 18:361–367
Healy JB, Young LY (1978) Catechol and phenol degradation by a methanogenic population of bacteria. Appl Environ Microbiol 35:216–218
Healy JB, Young LY (1979) Anaerobic biodegradation of eleven aromatic compounds to methane. Appl Environ Microbiol 38:84–89
Huser B, Wuhrmann K, Zehnder AJB (1982) Methanothrix soehngenii gen. nov., sp. nov., a new acetotrophic non-hydrogen oxidizing methane bacterium. Arch Microbiol 132:1–9
Imhoff D, Andreesen JR (1979) Nicotinic acid hydroxylase from Clostridium barkeri: selenium dependent formation of active enzyme. FEMS Microbiol Lett 5:155–158
Kupfer D (1964) Qualitative method for partial characterization of indole derivatives. Analyt Biochem 8:75–81
Mah RA, Smith MR (1981) The methanogenic bacteria. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The prokaryotes, vol I. Springer, Berlin Heidelberg New York, pp 948–977
Marmur J, Doty P (1962) Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol 5:109–118
Mead GC (1971) The amino acid-fermenting clostridia. J Gen Microbiol 67:47–56
Mountfort DO, Bryant MP (1982) Isolation and characterization of an anaerobic syntrophic benzoate-degrading bacterium from sewage sludge. Arch Microbiol 133:249–256
Schink B, Pfennig N (1982) Fermentation of trihydroxybenzenes by Pelobacter acidigallici gen. nov., sp. nov., a new strictly anaerobic, non-sporeforming bacterium. Arch Microbiol 133:195–201
Scott TW, Ward PFV, Dawson RMC (1964) The formation and metabolism of phenyl-substituted fatty acids in the ruminant. Biochem J 90:12–24
Sleat R, Robinson JP (1984) The bacteriology of anaerobic degradation of aromatic compounds. J Appl Bacteriol 57:381–394
Wagner R, Andreesen JR (1977) Differentiation between Clostridium acidiurici and Clostridium cylindrosporum on the basis of specific metal requirements for formate dehydrogenase formation. Arch Microbiol 121:255–260
Wang Y-T, Suidan MT, Pfeffer JT (1984) Anaerobic biodegradation of indole to methane. Appl Environ Microbiol 48:1058–1060
Widdel F (1983) Methods for enrichment and pure culture isolation of filamentous gliding sulfate-reducing bacteria. Arch Microbiol 134:282–285
Widdel F (1986) Microbiology and ecology of sulfate-and sulfurreducing bacteria. In: Zehnder AJB (ed) Environmental microbiology of anaerobes, chapter 10. John Wiley, New York London (in press)
Widdel F, Pfennig N (1981) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol 129:395–400
Widdel F, Pfennig N (1984) Dissimilatory sulfate-or sulfur-reducing bacteria. In: Krieg NR, Holt JG (eds) Bergey's manual of systematic bacteriology, IX. edn, vol I. Williams & Wilkins, Baltimore London, pp 663–679
Widdel F, Kohring GW, Mayer F (1983) Entides on dissimilatory sulfate-reducing bacteria that decompose fatty acids. III. Characterization of the filamentous gliding Desulfonema limicola gen. nov., sp. nov., and Desulfonema magnum sp. nov. Arch Microbiol 134:286–294
Yokoyama MT, Carlson JR (1974) Dissimilation of tryptophane and related indolic compounds by ruminal microorganisms in vitro. Appl Microbiol 27:540–548
Young LY (1984) Anaerobio degradation of aromatic compounds. In: Gibson DT (ed) Microbial degradation of organic compounds. Marcel Dekker, New York, pp 487–523
Young LY, Rivera MO (1985) Methanogenic degradation of four phenolic compounds. Water Res 19:1325–1332
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bak, F., Widdel, F. Anaerobic degradation of indolic compounds by sulfate-reducing enrichment cultures, and description of Desulfobacterium indolicum gen. nov., sp. nov.. Arch. Microbiol. 146, 170–176 (1986). https://doi.org/10.1007/BF00402346
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00402346