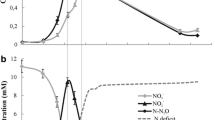
Abstract
Nitrous oxide reduction can consistently be demonstrated with high activities in cells of Azospirillum brasilense Sp 7 which are grown anaerobically in the presence of low amounts of nitrite. Azospirillum can even grow anaerobically with nitrous oxide in the absence of any other respiratory electron acceptor. Nitrous oxide reduction by Azospirillum is inhibited by acetylene, amytal and weakly by carbon monoxide. Azospirillum converts nitrous oxide to molecular nitrogen without the formation of ammonia. The cells must, therefore, be supplied with ammonia from nitrogen fixation during anaerobic growth with nitrous oxide. When no other nitrogen compound besides nitrous oxide is available in the medium, the bacteria synthesize nitrogenase from protein reserves in about 2 h. Nitrogenase synthesis is blocked by chloramphenicol under these conditions. In contrast, the addition of nitrate or nitrite to the medium represses the synthesis of nitrogenase. Nitrous oxide reduction by Azospirillum and other microorganisms is possibly of ecological significance, because the reaction performed by the bacteria may remove nitrous oxide from soils.
Similar content being viewed by others
References
Bothe H, Klein B, Stephan MP, Döbereiner J (1981), Transformations of inorganic nitrogen by Azospirillum spp. Arch Microbiol 130:96–100
Bothe H, Kronenberg A, Stephan MP, Zimmer W, Neuer G (1983a) Nitrogen fixation and denitrification by a wheat-Azospirillum association. In: Klingmüller W (ed) Azospirillum II, genetics, physiology, ecology. Experientia Suppl. 48. Birkhäuser, Basel, pp 100–113
Bothe H, Barbosa G, Döbereiner J (1983b) Nitrogen fixation and nitrate respiration by Azospirillum brasilense Sp 7. Z Naturforsch 38c:571–577
Bryan BA (1981) Physiology and biochemistry of denitrification. In: Delwiche CC (ed) Denitrification, nitrification and atmospheric nitrous oxide. Wiley, New York Chichester Brisbane Toronto, pp 67–84
Daniel RM, Smith IM, Phillip JAD, Ratcliffe HD, Drozd JW, Bull AT (1980) Anaerobic growth and denitrification by Rhizobium japonicum and other rhizobia. J Gen Microbiol 120:517–521
Döbereiner J (1983) Dinitrogen fixation in rhizosphere and phyllosphere associations. In: Läuchli A, Bieleski R (eds) Encyclopedia of plant physiology, New Series, vol 15A. Springer, Berlin Heidelberg New York, pp 330–350
Döbereiner J, De-Polli H (1980) Diazotrophic rhizocoenoses. In: Stewart WDP, Gallon JR (eds) Nitrogen fixation. Academic Press, London, pp 301–333
Gauthier D, Elmerich C (1977) Relationship between glutamine synthetase and nitrogenase in Spirillum lipoferum FEMS Microbiol Lett 2:101–104
Hardy RWF, Burns RC (1968) Biological nitrogen fixation. Ann Rev Biochem 37:331–358
Ingraham JL (1981) Microbiology and genetics of denitrifiers. In: Delwiche CC (ed) Denitrification, nitrification and atmospheric nitrous oxide. Wiley, New York Chichester Brisbane Toronto, pp 45–66
Landolt-Börnstein R (1923) Physikalisch-chemische Tabellen, V. Auflage. Springer, Berlin, pp 763–772
Matsubara T (1975) The participation of cytochromes in the reduction of N2O to N2 by a denitrifying bacterium. J Biochem Tokyo 77:627–632
Neal JL, Allen GC, Morse RD, Wolf DD (1983) Nitrate, nitrite, nitrous oxide and oxygen-dependent hydrogen uptake by Rhizobium FEMS Microbiol. Letters 17:335–338
Rigaud J, Bergersen FJ, Turner GL, Daniel, RM (1973) Nitrate dependent anaerobic acetylene reduction and nitrogen fixation by soybean bacteroids. J Gen Microbiol 77:137–144
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Yoshinari T (1980) N2O reduction by Vibrio succinogenes. Appl Environm Microbiol 39:81–84
Zimmer W, Stephan MP, Bothe H (1984) Denitrification by Azospirillum brasilense Sp 7. I. Growth with nitrite as respiratory electron acceptor. Arch Microbiol 138:206–211
Zumft WG, Matsubara T (1982) A novel kind of multi-copper protein as terminal oxidoreductase of nitrous oxide respiration in Pseudomonas perfectomarinus. FEBS Letters 148:107–111
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stephan, M.P., Zimmer, W. & Bothe, H. Denitrification by Azospirillum brasilense Sp 7. Arch. Microbiol. 138, 212–216 (1984). https://doi.org/10.1007/BF00402122
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00402122