Summary
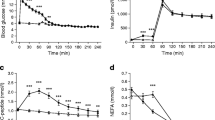
To examine determinants of basal metabolic rate we studied 66 Type 2 (non-insulin-dependent) diabetic and 24 healthy age- and weight-matched control subjects with indirect calorimetry and infusion of [3H-3-] glucose. Eight Type 2 diabetic patients were re-studied after a period of insulin therapy. Basal metabolic rate was higher in Type 2 diabetic patients than in control subjects (102.8 ± 1.9 J · kg LBM−1-min−1 vs 90.7 ± 2.8 J · kg LBM−1;min−1; p<0.01) and decreased significantly with insulin therapy (p <0.01). The basal rate of hepatic glucose production was higher in Type 2 diabetic patients than in control subjects (1044.0 ± 29.9 vs 789.3 ± 41.7 μmol/min; p <0.001) and decreased after insulin therapy (p <0.01). Hepatic glucose production correlated positively with basal metabolic rate both in Type 2 diabetic patients (r = 0.49; p <0.001) and in control subjects (r = 0.50; p<0.05). Lipid oxidation was increased in Type 2 diabetic patients compared with control subjects (1.68 ± 0.05 vs 1.37 ± 0.08 μmol · kg LBM−1 · min−1'; p <0.01) and decreased significantly after insulin therapy (p <0.05). The rate of lipid oxidation correlated positively with basal metabolic rate both in Type 2 diabetic patients (r = 0.36; p <0.01) and in control subjects (r = 0.51; p <0.01). These data demonstrate that basal metabolic rate, rates of hepatic glucose production and lipid oxidation are interrelated in Type 2 diabetic patients. A reduction of the hepatic glucose production, however, is associated with a reduction in lipid oxidation, which in turn, may result in a reduction in basal metabolic rate.
Article PDF
Similar content being viewed by others
References
Bogardus C, Taskinen MR, Zawadzki J, Lillioja S, Mott D, Howard B (1986) Increased resting metabolic rate in obese subjects with non-insulin-dependent diabetes mellitus and the effect of sulfonylurea therapy. Diabetes 35: 1–5
Goldner MG, Knaterud GL, Prout TE (1971) Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes mellitus. III. Clinical implications of UGDP results. JAMA 218: 1400–1410
Geldermans CA, Terpstra J, Krans HMJ (1975) The effect of phenformin-HCL on patients with diabetes mellitus, studied under strict balance conditions. Diabetologia 11: 475–484
DeFronzo RA (1988) The triumvirate: β-cell, muscle, liver: a collusion responsible for NIDDM. Diabetes 37: 667–683
Consoli A, Nurjhan N, Capani F, Gerich J (1989) Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes 38: 550–557
Ruderman NC, Toews JD, Shafrir E (1969) Role of free fatty acids in glucose homeostasis. Arch Intern Med 123: 299–313
Bogardus C, Lillioja S, Howard BV, Reaven G, Mott D (1984) Relationship between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin dependent diabetic subjects. J Clin Invest 74: 1238–1246
Blumenthal SA (1983) Stimulation of gluconeogenesis by palmitic acid in rat hepatocytes: evidence that this effect can be dissociated from the provision of reducing equivalents. Metab Clin Exp 32: 971–976
Golay A, Schutz Y, Felber JP, DeFronzo RA, Jéquier E (1986) Lack of thermogenic response to glucose/insulin infusion in diabetic obese subjects. Int J Obesity 10: 107–116
Ravussin E, Burnand B, Schutz Y, Jequier E (1982) Twenty-four-hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects. Am J Clin Nutr 35: 566–573
Ferrannini E (1988) The theoretical basis for indirect calorimetry: a review. Metabolism 37: 287–301
Coleman TG, Manning RD Jr, Norman RA Jr, Guyton AC (1972) Dynamics of water-isotope distribution. Am J Physiol 223: 1371–1375
Miles JR, Glasscock J, Aikens J, Gerich J, Raymond M (1983) A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res 24: 96–99
Ravussin E, Bogardus C, Schwartz RS et al. (1983) Thermic effect of infused glucose and insulin in man. J Clin Invest 72: 893–902
Williamson JR, Kreisberg RA, Felts PW (1966) Mechanisms for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci USA 6: 247–254
Garland PB, Randle P (1964) Control of pyruvate-dehydrogenase in the perfused rat heart by the intracellular concentration of acetyl Co A. Biochem J 91: 6C-7C
Groop LC Bonadonna RC, DelPrato S et al. (1989) Glucose and free fatty acid metabolism in non-insulin dependent diabetes mellitus: evidence for multiple sites of insulin resistance. J Clin Invest 84: 205–213
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Franssila-Kallunki, A., Groop, L. Factors associated with basal metabolic rate in patients with Type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 35, 962–966 (1992). https://doi.org/10.1007/BF00401426
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00401426