Abstract
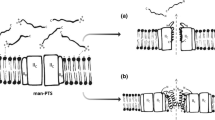
Bacteria producing bacteriocins have to be protected from being killed by themselves. This mechanism of self-protection or immunity is especially important if the bacteriocin does not need a specific receptor for its action, as is the case for the type A lantibiotics forming pores in the cytoplasmic membrane. At least two different systems of immunity have evolved in this group of bacteriocins containing modified amino acids as a result of posttranslational modification. The immunity mechanism of Pep5 in Staphylococcus epidermidis is based on inhibition of pore formation by a small 69-amino acid protein weakly associated with the outer surface of the cytoplasmic membrane. In Lactococcus lactis and Bacillus subtilis the putative immunity lipoproteins NisI and SpaI, respectively, are also located at the outer surface of the cytoplasmic membrane, suggesting that a similar mechanism might be utilized by the producers of nisin and subtilin. In addition an ABC-transport system consisting of two membrane proteins, (NisEG, SpaG and the hydrophobic domain of SpaF, and EpiEG) and a cytoplasmic protein (NisF, the cytoplasmic domain of SpaF, and EpiF) play a role in immunity of nisin, subtilin and epidermin by import, export or inhibition of pore formation by the membrane components of the transport systems. Almost nothing is known of the immunity determinants of newly described and other type of lantibiotics.
Similar content being viewed by others
References
Banerjee S & Hansen JN (1988) Structure and expression of a gene encoding the precursor of subtilin, a small protein antibiotic. J. Biol. Chem. 262: 9508–9514
Bierbaum G, Götz F, Peschel A, Kupke T, Kamp M van der & Sahl H-G (1995) The biosynthesis of the lantibiotics epidermin, gallidermin, Pep5 and Epilancin K7. Antonie van Leeuwenhoek (this volume)
Bowman CM, Sidikara J & Nomura M (1971) Specific inactivation of ribosomes by colicin E3 in intro and mechanism of immunity in colicinogenic cells. Nature 48: 133–137
Buchman GW, Banerjee S & Hansen JN (1988) Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J. Biol. Chem. 264: 16260–16266
Chung YJ, Steen MT & Hansen JN (1992) The subtilin gene of Bacillus subtilis ATCC 6633 is encoded in an operon that contains a homolog of the hemolysin B transport protein. J. Bacteriol. 174: 1417–1422
Dodd HM, Horn N & Gasson MJ (1990) Analysis of the genetic determinant for production of the peptide antibiotic nisin. J. Gen. Microbiol. 136: 555–566
Dodd HM, Horn N, Hao Z & Gasson MJ (1992) A lactococcal expression system for engineered nisins. Appl. Environ. Microbiol. 58: 3683–3693
Engelke G, Gutowski-Eckel Z, Hammelmann M & Entian K-D (1992) Biosynthesis of the lantibiotic nisin: genomic organization and membrane localization of the NisB protein. Appl. Environ. Microbiol. 58: 3730–3743
Engelke G, Gutowski-Eckel Z, Kiesau P, Siegers K, Hammelman M & Entian K-D (1994) Regulation of nisin biosynthesis and immunity in Lactococcus lactis 6F3. Appl. Environ. Microbiol. 60: 814–825
Ersfeld-Dreßen H, Sahl H-G & Brandis H (1984) Plasmid involvement in production of and immunity to the staphylococcin-like peptide Pep5. J. Gen. Microbiol. 130: 3029–3035
Gasson MJ (1984) Transfer of sucrose fermenting ability, nisin resistance and nisin production into Streptococcus lactis 712. FEMS Microbiol. Lett. 21: 7–10
Garrido MC, Herrero M, Kolter R & Moreno F (1988) The export of the DNA replication inhibitor microcin B17 provides immunity for the host cell. EMBO J. 7: 1853–1862
Gilmore MS, Segarra RA & Booth MC (1990) A HlyB-type function is required for expression of the Enterococcus faecalis hemolysin/bacteriocin. Infect. Immun. 58: 3914–3923
Gilson E, Alloing G, Schmidt T, Claverys J-P, Dudler R & Hofnung M (1988) Evidence for high affinity binding-protein dependent transport systems in Gram-positive bacteria and in Mycoplasma. EMBO J. 7: 3971–3974
Graeffe T, Rintala H, Paulin L & Saris P (1991) A natural nisin variant. In: Jung C & Sahl H-G (Eds) Nisin and Novel Lantibiotics. (pp 260–268) ESCOM Leiden
Horn N, Swindell S, Dodd H & Gasson M (1991) Nisin biosynthesis genes are encoded by a novel conjugative transposon. Mol. Gen. Genet. 228: 129–135
Hynes WL, Ferretti JJ & Tagg JR (1993) Cloning of the gene encoding streptococcin A-FF22, a novel lantibiotic produced by Streptococcus pyogenes, and determination of its nucleotide sequence. Appl. Environ. Microbiol. 59: 1961–1971
Immonen Y, Ye S, Ra R, Qiao M, Paulin L & Saris PEJ (1995) The codon usage of the nisin Z operon in Lactococcus lactis N8 suggests a non-lactococcal origin of the conjugative nisin-sucrose transposon. Sequence 5: 203–218
Kellner R, Jung G, Hörner T, Zähner H, Schell N, Entian K-D & Götz F (1988) Gallidermin: a new lanthionine-containing polypeptide antibiotic. Eur. J. Biochem. 177: 53–59
Klein C & Entian K-D (1994) Genes involved in self-protection anainst the lantibiotic subtilin produced by Bacillus subtilis ATCC6633. Appl. Environ. Microbiol. 60: 2793–2801
Klein C, Kaletta C, Schnell N & Entian K-D (1992) Analysis of genes involved in biosynthesis of the lantibiotic subtilin. Appl. Environ. Microbiol. 58: 132–142
Klein C, Kaletta C & Entian K-D (1993) Biosynthesis of the lantibiotic subtilin is regulated by a histidine kinase/response regulator system. Appl. Environ. Microbiol. 59: 296–303
Kuipers OP, Beerthuyzen MM, Siezen RJ & Vos WMde (1993) Characterization of the nisin gene cluster nisABTCIPRK of Lactococcus lactis and evidence for the involvement of expression of the nisA and nisA genes in product immunity. Eur. J. Biochem. 216: 281–291
Lewis K (1994) Multidrug resistance pumps in bacteria: variations on a theme. TIBS 19: 119–123
Meyer C, Bierbaum G, Heidrich C, Reis M, Süling J, Iglesias-Wind M, Kempter C, Molitor E & Sahl H-G (1995) Nucleotide sequence of the lantibiotic Pep5 biosynthetis cluster, functional analysis of Pep% and PepC and evidence for a role of PepC in thioether formation. Eur. J. Biochem. (in press)
Mulders JWM, Boerrighter IJ, Rollema HS, Siezen RJ & Vos WMde (1991) Identification and characterization of the lantibiotic nisin Z, a natural nisin variant. Eur. J. Biochem. 201: 581–584
Nissen-Mayer J, Havarstein LS, Holo H, Sletten K & Nes IF (1993) Association of the lactococcin A immunity factor with the cell membrane: purification and characterization of the immunity factor. J. Gen. Microbiol. 139: 1503–1522
Mørtvedt CI, Nissen-Mayer J, Sletten K & Nes IF (1991) Purificiation and amino acid sequence of lactocin S, a bacteriocin produced by Lactobacillus sake L45. Appl. Environ. Microbiol. 57: 1829–1834
Novak J, Caufield PW & Miller EJ (1994) Isolation and biochemical characterization of a novel lantibiotic mutacin from Streptococcus mutant. J. Bacteriol. 176: 4316–4320
Quadri LEN, Sailer M, Terebiznik MR, Roy KL, Vederas JC & Stiles ME (1995) Characterization of the protein conferring immunity to the antimicrobial peptide Carnobacteriocin B2 and expression of Carnobacteriocins B2 and BM1. J. Bacteriol. 177: 1144–1151
Reis M & Sahl H-G (1991) Genetic analysis of the producer self protection mechanism (‘immunity’) against Pep5. In: Jung G & Sahl H-G (Eds) Nisin and Novel Lantibiotics. (pp 320–332) ESCOM Leiden
Reis M, Eschblach-Bludau M, Inglesias-Wind MI, Kupke T & Sahl H-G (1994) Producer immunity towards the lantibiotic Pep5: identification of the immunity gene pepI and localization and functional analysis of its gene product. Appl. Environ. Microbiol. 60: 2867–2883
Rintala H, Graeffe T, Paulin L, Kalkkinen N & Saris PEJ (1993) Biosynthesis of nisin in the subtilin producer Bacillus subtilis ATCC6633. Biotechnology Lett. 15: 991–996
Rauch PJG & Vos WMde (1992) Characterization of the novel nisin-sucrose conjugative transposon Tn5267 and its insertion in Lactococcus lactis. J. Bacteriol. 174: 1280–1287
Rince A, Dufour A, LePogam S, Thuault D, Bourgeois CM & LePennec JP (1994) Cloning, expression, and nucleotide sequence of genes involved in the production of lactococcin DR, a bacteriocin from Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 60: 1652–1657
Ross KF, Ronson WC & Tagg JR (1993) Isolation and characterization of the lantibiotic salvaricin A and its structural gene salA from Streptococcus salvarius 20P3. Appl. Environ. Microbiol. 59: 2014–2021
Russell RRB, Aduse-Opoku J, Sutcliffe IC, Tao L & Ferretti JJ (1992) A binding protein-dependent transport system in Streptococcus mutans responsible for multiple sugar metabolism. J. Biol. Chem. 267: 4631–4637
Sahl H-G (1994) Staphylococcin 1580 is identical to the lantibiotic epidermin: implications for the nature of bacteriocins from grampositive bacteria. Appl. Environ. Microbiol. 60: 752–755
Saier MHJr (1994) Computer-aided analyses of transport protein sequences: Gleaning evidence concerning function, structure, biogenesis, and evolution. Microbiol. Rev. 58: 71–93
Schnell N, Engelke G, Augustin J, Rosenstein R, Ungerman V, Götz F & Entian K-D (1992) Analysis of genes involved in the biosynthesis of the lantibiotic epidermin. Eur. J. Biochem. 204: 57–68
Sibakov M, Koivula T, Wright Avon & Palva I (1991) Secretion of TEM β-lactamase with signal sequences isolated from the chromosome of Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 57: 341–348
Siegers K & Entian K-D (1995) Genes involved in immunity to the lantibiotic nisin produced by Lactococcus lactis 6F3. Appl. Environ. Microbiol. 61: 1082–1089
Skaugen M (1994) Lactocin S: structure determination and genetic analysis. PhD thesis, As, Agricultural University of Norway
Song H-Y & Cramer WA (1991) Membrane topology of ColE1 gene products: The immunity protein. J. Bacteriol. 173: 2935–2943
Steen MT, Chung YJ & Hansen JN (1991) Characterization of the nisin gene as part of a polycistronic operon in the chromosome of Lactococcus lactis. Appl. Environ. Microbiol. 57: 1181–1188
Stoffels G, Nissen-Mayer J, Gudmundsdottir A, Sletten K, Helge H & Nes IF (1992) Purification and characterization of a new bacteriocin from a Carnobacterium sp. Appl. Environ. Microbiol. 58: 1417–1422
Sutcliffe IC, Tao L, Ferretti JJ & Russell RR (1993) MsME, a lipoprotein involved in sugar transport in Streptococcus mutans. J. Bacteriol. 175: 1853–1855
Tynkkynen S, Buist G, Kunij E, Kok J, Poolman B, Venem G & Haandrikman A (1993) Genetic and biochemical characterization of the oligopeptide transport system of Lactococcus lactis. J. Bacteriol. 175: 7523–7532
Vos WMde, Mulders JWM, Hugenholz J, Siezen RJ & Kuipers OP (1993) Properties of nisin Z and distribution of its gene, nisZ, in Lactococcus lactis. Appl. Environ. Microbiol. 59: 213–218
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Joakim Saris, P.E., Immonen, T., Reis, M. et al. Immunity to lantibiotics. Antonie van Leeuwenhoek 69, 151–159 (1996). https://doi.org/10.1007/BF00399420
Issue Date:
DOI: https://doi.org/10.1007/BF00399420