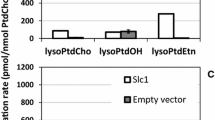
Abstract
Peroxisomes from mung-bean hypocotyls catalyze, in the presence of fatty acids, CoASH, ATP, and MgCl2, the formation of acyl-CoA, AMP, and pyrophosphate in a 1:1:1 stoichiometry. This observation demonstrates that the peroxisomes of mung-bean hypocotyls possess an acyl-CoA synthetase (EC 6.2.1.3) for fatty-acid activation. Acyl-CoA synthetase activity is associated with the non-glyoxysomal peroxisomes from various tissues. The acyl-CoA synthetase of the peroxisomes of the mung-bean hypocotyl utilizes oleic, linoleic, and linolenic acid most effectively (3 nkat·mg-1 peroxisomal protein). In contrast to the β-oxidation enzymes of the peroxisomes whith are largely solubilized in the presence of 0.2 mol·l-1 KCl, the acyl-CoA synthetase remains associated with the membrane fraction of peroxisomes. On the basis of the latency of the enzyme and its resistance to protease treatment of the peroxisomes, it is concluded that the enzyme is located at the matrix face of the peroxisome membrane.
Similar content being viewed by others
Abbreviations
- EGTA:
-
ethyleneglycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
References
Andrews, J., Keegstra, K. (1983) Acyl-CoA synthetase is located in the outer membrane and acyl-CoA thioesterase in the inner membrane of pea chloroplast envelopes. Plant Physiol. 72, 735–740
Bergmeyer, H.U., Gawehn, K., Graßl, M. (1974) Enzyme als biochemische Reagentien. In: Methoden der enzymatischen Analyse, pp. 454–558, Bergmeyer, H.U., ed. Verlag Chemie, Weinheim
Block, M.A., Dorne, A.-J., Joyard, J., Douce, R. (1983) The acyl-CoA synthetase and acyl-CoA thioesterase are located on the outer and inner membrane of the chloroplast envelope, respectively. FEBS Lett. 153, 377–381
Cooper, T.C. (1971) The activation of fatty acids in castor bean endosperm. J. Biol. Chem. 246, 3451–3455
Douce, R., Joyard, J. (1979) Structure and function of the plastid envelope. Adv. Bot. Res. 7, 1–116
Edwards, J., ap Rees, T., Wilson, P.M., Morrel, S. (1984) Measurement of the inorganic pyrophosphate in tissues of Pisum sativum L. Planta 162, 188–191
Eising, R., Gerhardt, B. (1986) Activity and hematin content of catalase from greening sunflower cotyledons. Phytochemistry 25, 27–31
Gerhardt, B. (1983) Localization of β-oxidation enzymes in peroxisomes isolated from nonfatty plant tissues. Planta 159, 238–246
Gerhardt, B. (1986) Basic metabolic function of the higher plant peroxisome. Physiol. Vég. 24, 397–410
Gerhardt, B. (1987a) Peroxisomes and fatty acid degradation. Methods Enzym. in press
Gerhardt, B. (1987b) Higher plant peroxisomes and fatty acid degradation. In: Peroxisomes in biology and medicine, pp. 141–151, Fahimi, D., Sies, H., eds. Springer, Berlin Heidelberg New York
Hashimoto, T. (1982) Individual peroxisomal β-oxidation enzymes. Ann. N.Y. Acad. Sci. 386, 5–12
Joyard, J., Billecocq, A., Bartlett, S.G., Block, M.A., Chua, N.-H., Douce R. (1983) Localization of polypeptides to the cytosolic side of the outer envelope membrane of spinach chloroplasts. J. Biol. Chem. 258, 10 000–10 006
Joyard, J., Stumpf, P.K. (1981) Synthesis of long-chain acyl-CoA in chloroplast envelope membranes. Plant Physiol. 67, 250–256
Kow, Y.W., Erbes, D.L., Gibbs, M. (1982) Chloroplast respiration. A means of supplying oxidized pyridine nucleotide for dark chloroplastic metabolism. Plant Physiol. 69, 442–447
Krisans, S.K., Mortensen, R.M., Lazarow, P.B. (1980) Acyl-CoA synthetase in rat liver peroxisomes. J. Biol. Chem. 255, 9599–9607
Leusing, P. (1985) Photosynthetische 14C-Markierung von Aminosäuren in reifenden Weizenpflanzen, Transport und Einbau in Kornproteine. Dissertation, Universität Münster
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275
Mannaerts, G.P., van Veldhoven, P., van Broekhoven, A., Vandebroek, G., Debeer, L.J. (1982) Evidence that peroxisomal acyl-CoA synthetase is located at the cytoplasmic side of the peroxisomal membrane. Biochem. J. 204, 17–23
Miernyk, J.A., Trelease, R.N. (1981) Control of enzyme activities in cotton cotyledons during maturation and germination. IV. β-Oxidation. Plant Physiol. 67, 341–346
Murphy, D.J., Mukherjee, K.D., Latzko, E. (1983) Lipid metabolism in microsomal fractions from photosynthetic tissue. Biochem. J. 213, 249–252
Pryde, J.G., Phillips, J.H. (1986) Fractionation of membrane proteins by temperature-induced phase separation in Trition X-114. Application to subcellular fractions of the adrenal medulla. Biochem. J. 233, 525–533
Randall, P.J., Bouma, D. (1973) Zinc deficiency, carbonic anhydrase, and photosynthesis in leaves of spinach. Plant Physiol. 52, 229–232
Roughan, P.G., Slack, C.R. (1977) Long-chain acyl-coenzyme A synthetase activity of spinach chloroplasts is concentrated in the envelope. Biochem. J. 162, 457–459
Schmidt, E. (1974) Glutamat-Dehydrogenase. In: Methoden der enzymatischen Analyse, pp. 689–696, Bergmeyer, H.U., ed. Verlag Chemie, Weinheim
Schnarrenberger, C., Oeser, A., Tolbert, N.E. (1971) Development of microbodies in sunflower cotyledons and castor bean endosperm during germination. Plant Physiol. 48, 566–574
Schuh, B., Gerhardt, B. (1984) Size of microbody population in sunflower cotyledons during the transition in cotyledonary microbody function. Z. Pflanzenphysiol. 114, 477–484
Tanaka, T., Hosaka, K., Hoshimaru, M., Numa, S. (1979) Purification and properties of long-chain acyl-coenzyme-A synthetase from rat liver. Eur. J. Biochem. 98, 165–172
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gerbling, H., Gerhardt, B. Activation of fatty acids by non-glyoxysomal peroxisomes. Planta 171, 386–392 (1987). https://doi.org/10.1007/BF00398684
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00398684