Abstract
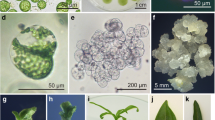
Protoplasts isolated from Nicotiana tabacum L. leaves and Nicotiana suaveolens Lehm. cell suspensions have been fused with polyethylene glycol (PEG). Enrichment for heterokaryons was based on a Percoll flotation protocol which allowed a preparation with 50% heterokaryons to be obtained. The heterokaryons developed into calli whose hybrid nature was shown by polyacrylamide gel electrophoresis of esterase isoenzymes. Sensitivity of the mesophyll protoplasts to PEG and different buoyant densities of the heterokaryon and cell-suspension protoplasts contribute to the enrichment. The 50%-fusion figure following purification is an improvement on standard PEG procedures.
Heterokaryons obtained were embedded in 20μl drops of agarose and placed in a liquid nurse culture that allows optimum growth of the heterokaryons and maintains a physical boundary between the heterokaryons and the nurse culture. Once colonies develop, the agarose microdrop is removed from the nurse culture and placed on shoot-induction medium. Agarose microdrops containing the heterokaryons can be readily removed at any stage and processed for electron microscopy to follow the early stages of colony development.
The procedures we have utilised provide a robust physical selection method that allows the total variation from a heterokaryon population to be expressed.
Similar content being viewed by others
Abbreviations
- BAP:
-
N6-benzylaminopurine
- BM:
-
basal medium
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- NAA:
-
1-naphthalene acetic acid
- PEG:
-
polyethylene glycol
- PKM:
-
modified Kao (1977) medium for protoplast culture
References
Bates, G.W. (1985) Electrical fusion for optimal formation of protoplast heterokaryons in Nicotiana. Planta 165, 217–224
Bates, G.W., Hasenkampf, C. (1985) Culture of plant somatic hybrids following electrical fusion. Theor. Appl. Genet. 70, 227–233
Caboche, M., Aranda, G., Poll, A.M., Huet, J.-C., Leguay, J.-J. (1984) Auxin conjugation by tobacco mesophyll protoplasts. Correlations between auxin cytotoxicity under low density growth conditions and induction of conjugation processes at high density. Plant Physiol. 75, 54–59
Dalton, C.C., Iqbal, K., Turner, D.A. (1983) Iron phosphate precipitation in Murashige and Skoog media. Physiol. Plant. 57, 472–476
Douglas, G.C., Keller, W.A., Setterfield, G. (1981) Somatic hybridisation between Nicotiana rustica and N. tabacum. II. Protoplast fusion and selection and regeneration of hybrid plants. Can. J. Bot. 59, 220–227
Flick, C.E., Kut, S.A., Bravo, J.E., Gleba, Y.Y., Evans, D.A. (1985) Segregation of organelle traits following protoplast fusion in Nicotiana Bio/Technology 3, 559–560
Gamborg, O.L., Miller, R.A., Ojima, K. (1968) Nutrient requirements of a suspension culture of soybean root cells. Exp. Cell Res. 50, 151–158
Gleba, Y.Y. (1978) Microdroplet culture. Tobacco plants from mesophyll protoplasts. Naturwissenschaften. 65, 158–159
Gleba, Y.Y., Sytnik, K.M. (1984) Protoplast fusion, genetic engineering in higher plants. Springer Verlag, Berlin
Gleba, Y.Y., Kolesnik, N.N., Meshkene, I.V., Cherep, N.N., Parokonny, A.S. (1984) Transmission genetics of the somatic hybridisation in Nicotiana. I. Hybrids and cybrids among the regenerates from cloned protoplast fusion products. Theor. Appl. Genet. 69, 121–128
Hamill, J.D., Patnaik, G., Pental, D., Cocking, E.C. (1984) The culture of manually isolated heterokaryons of Nicotiana tabacum and Nicotiana rustica. Proc. Indian Acad. Sci. (Plant Sci.) 93, 317–328
Harms, C.T., Potrykus, I. (1978) Enrichment for heterokaryocytes by the use of iso-osmotic density gradients after plant protoplast fusion. Theor. Appl. Genet. 53, 49–55
Hein, T., Przewozny, T., Schieder, O. (1983) Culture and selection of somatic hybrids using an auxotrophic cell line. Theor. Appl. Genet. 64, 119–122
Hodgson, R.A.J., Rose, R.J. (1984) Fusion of spinach mesophyll protoplasts with carrot root parenchyma protoplasts and the effect on spinach chloroplasts. J. Plant Physiol. 115, 69–78
Kamata, Y., Nagata, T. (1987) Enrichment of heterokaryocytes between mesophyll and epidermis protoplasts by density gradient centrifugation after electric fusion. Theor. Appl. Genet. 75, 26–29
Kao, K.N. (1977) Chromosomal behaviour in somatic hybrids of soybean-Nicotiana glauca. Mol. Gen. Genet. 150, 225–230
Kao, K.N. (1986) Fusion of plant protoplasts at the interface of a glucose and a sucrose-polyethylene glycol solution. J. Plant Physiol. 126, 55–58
Kao, K.N., Michayluk, M.R. (1974) A method for high-frequency intergeneric fusion of plant protoplasts. Planta 115, 355–367
Kao, K.N., Saleem, M. (1986) Improved fusion of mesophyll and cotyledon protoplasts with PEG and high pH-Ca2+ solutions. J. Plant Physiol. 122, 217–225
Koop, H.U., Schweiger, H.G. (1985) Regeneration of plants after electrofusion of selected pairs of protoplasts. Eur. J. Cell Biol. 39, 46–49
Koop, H.U., Weber, G., Schweiger, H.G. (1983) Individual culture of selected single cells and protoplasts of higher plants in microdroplets of defined media. Z. Pflanzenphysiol. 112, 21–34
Lawrence, W.A., Davies, D.R. (1985) A method for the micro-injection and culture of protoplasts at very low densities. Plant Cell Rep. 4, 33–35
Medgyesy, P., Menczel, L., Maliga, P. (1980) The use of cytoplasmic streptomycin resistance: Chloroplast transfer from Nicotiana tabacum into Nicotiana sylvestris and isolation of their somatic hybrids. Mol. Gen. Genet. 179, 693–698
Menczel, L., Lazar, G., Maliga, P. (1978) Isolation of somatic hybrids by cloning Nicotiana heterokaryons in nurse culture. Planta 143, 29–32
Menczel, L., Wolfe, K. (1984) High frequency of fusion induced in freely suspended protoplast mixtures by polyethylene glycol and dimethylsulfoxide at high pH. Plant Cell Rep. 3, 196–198
Murashige, T., Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 57, 473–497
Schweppenhauser, M.A., Mann, T.J. (1968) Restoration of staminal fertility in Nicotiana by introgression. Can. J. Genet. Cytol. 10, 401–411
Shneyour, Y., Zelcer, A., Izhar, S., Beckman, J.S. (1984) A simple feeder-layer technique for the plating of plant cells and protoplasts at low density. Plant Sci. Lett. 33, 293–302
Tempelaar, M.J., Jones, M.G.K. (1985) Directed electrofusion between protoplasts with different responses in a mass fusion system. Plant Cell Rep. 4, 92–95
Thomas, M.R., Rose, R.J. (1983) Plastid number and plastid structural changes associated with tobacco mesophyll protoplast culture and plant regeneration. Planta 158, 329–338
Zimmermann, U., Scheurich, P. (1981) High frequency fusion of plant protoplasts by electric fields. Planta 151, 26–32
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thomas, M.R., Rose, R.J. Enrichment for Nicotiana heterokaryons after protoplast fusion and subsequent growth in agarose microdrops. Planta 175, 396–402 (1988). https://doi.org/10.1007/BF00396346
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00396346