Abstract
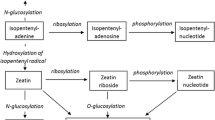
Post-embedding immunocytochemical techniques using peroxidase-antiperoxidase or immunoglobulin G-gold as markers were used for the localization of cytokinins (CKs) in two isogenic lines, Craigella (C) and Craigella lateral suppressor (Cls), of tomato Lycopersicon esculentum Mill. Terminal buds, nodes, hypocotyl segments and root tips were submitted to a periodate-borohydride procedure, to obtain the coupling of isopentenyladeosine and zeatin riboside to cellular proteins, followed by a fixative step with a paraformaldehyde and glutaraldehyde mixture. Enzyme-linked immunosorbent assay tests performed on ovalbumin-coated microtitration plates have shown that this method was effective for CK riboside and base coupling to proteins. Paraffin-wax- or Spurr's-resin-embedded sections were cleared of wax or resin before incubation with anti-zeatin riboside or anti-isopentenyladenosine antibodies. The procedure was thoroughly investigated and many controls were done in order to eliminate artefacts. The immunostaining patterns observed along the plants showed a basipetally decreasing gradient of CKs along the stem and in the roots. Immunolabelling was higher in the actively growing regions of the stem bud and root apices. Terminal buds of Cls appeared to be less immunoreactive than C, whereas no differences were detected in root-tip immunolabelling. The staining patterns are consistent with the idea that root and bud apices have a different CK metabolism. The absence of axillary bud formation in Cls is correlated with low CK levels in the organogensis sites.
Similar content being viewed by others
Abbreviations
- C:
-
Craigella, isogenic line
- CK:
-
cytokinin
- Cls:
-
Craigella lateral suppressor
- EDC:
-
1-(3-dimethylaminopropyl)3-ethylcarbodiimide hydrochloride
- ELISA:
-
enzyme-linked immunosorbent assay
- 2iP:
-
isopentenyladenine
- 2iPA:
-
isopentenyladenosine
- PAP:
-
peroxidase-anti-peroxidase
- PFAG:
-
paraformaldehyde/glutaraldehyde mixture
- Z:
-
zeatin
- ZR:
-
zeatin riboside
References
Cahill, D.M., Weste, G.M., Grant, B.R. (1986) Changes in cytokinin concentrations in xylem extrudate following infection of Eucalyptus marginata Donn ex Sm with Phytophthora cinnamomi rands. Plant Physiol. 81, 1103–1109
Chen, C.-M., Petschow, B. (1978) Cytokinin biosynthesis in cultured rootless tobacco plants. Plant Physiol. 62, 861–865
Childs, G.V. (1983) The use of multiple methods to validate immunocytochemical stains. J. Histochem. Cytochem. 31, 168–176
Childs, V., Unabia, G., Ellison, D. (1986) Immunocytochemical studies of pituitary hormones with PAP, ABC, and immunogold techniques: Evolution of technology of best fit the antigen. Am. J. Anat. 175, 307–330
De Waele, M., De Mey, J., Renmans, W., Labeur, E., Reynaert, Ph., Van Camp, B. (1986) Immunogold-silver staining of lymphocyte surface antigens on cells in suspension and in lymph node cryostat sections. J. Microsc. 143, 151–160
Eberle, J., Wang, T.L., Cook, S., Wells, B., Weiler, E.W. (1987) Immunoassay and ultrastructural localization of isopentenyladenine and related cytokinins using monoclonal antibodies. Planta 172, 289–297
Erlanger, B.F., Beiser, S.M. (1964) Antibodies specific for ribonucleosides and ribonucleotides and their reaction with DNA. Proc. Natl. Acad. Sci. USA. 52, 68–74
Hanker, J.S., Yates, P.E., Metz, C.B., Rustioni, A. (1977) A new specific, sensitive and non carcinogenic reagent for the demonstration of horse-radish peroxidase. Histochem. J. 9, 789–792
Holgate, C.S., Jackson, P., Cowen, P.N., Bird, C.C. (1983) Immunogold-silver staining. New method of immunostaining with enhanced sensitivity. J. Histochem. Cytochem. 31, 938–944
Koda, Y., Okazawa, Y. (1980) Cytokinin production by Asparagus shoot apex cultured in vitro. Physiol. Plant. 49, 193–197
Leroux, B., Maldiney, R., Miginiac, E., Sossountzov, L., Sotta, B. (1985) Comparative quantitation of abscisic acid in plant extracts by gas-liquid chromatography and an enzymelinked immunosorbent assay using the avidin-biotin systems. Planta 166, 524–529
Letham, D.S. (1978) Cytokinins. In: Phytohormones and related compounds: a comprehensive treatise, vol. 1, pp. 205–263, Letham, D.S., Goodwin, P.B., Higgins T.V.J., eds. Elsevier/North-Holland Biomedical Press, Amsterdam Oxford New York
Maldiney, R., Leroux, B., Sabbagh, I., Sotta, B., Sossountzov, L., Miginiac, E. (1986a) A biotin-avidin-based enzyme immunoassay to quantify three phytohormones: auxin, abscisic acid and zeatin-riboside. J. Immunol. Meth. 90, 151–158
Maldiney, R., Pelèse, F., Pilate, G., Sotta, B., Sossountzov, L., Miginiac, E. (1986b) Endogenous levels of abscisic acid, indole-3-acetic acid, zeatin and zeatin-riboside during the course of adventitous root formation in cuttings of Craigella and Craigella lateral suppressor tomatoes. Physiol. Plant. 68, 426–430
Mapelli, S., Lombardi, L. (1982) A comparative auxin and cytokinin study in normal and to-2 mutant tomato plants. Plant Cell Physiol. 23, 751–757
Mazia, D., Brewer, P.A., Alfert, M. (1953) THe cytochemical staining and measurement of protein with mercuric bromphenol blue. Biol. Bull. 104, 57–67
Palmer, M.V., Wong, O.C. (1985) Identification of cytokinins from xylem exudate of Phaseolus vulgaris L. Plant Physiol. 79, 296–298
Palmer, M.V., Horgan, R., Wareing, P.F. (1981) Cytokinin metabolism in Phaseolus vulgaris L. Identification of endogenous cytokinins and metabolism of [8-14C]dihydrozeatin in stems of decapitated plants. Planta 153, 297–302
Pengelly, W.L., Meins, F., Jr. (1977) A specific radioimmunoassay for nanogram quantities of the auxin, indole-3-acetic acid. Planta 136, 173–180
Petrusz, P. (1983) Essential requirements for the validity of immunocytochemical staining procedures. J. Histochem. Cytochem. 31, 177–179
Petrusz, P., Ordinneau, P., Finley, J.C.W. (1980) Criteria of reliability for light microscopic immunocytochemical staining. Histochem. J. 12, 333–348
Scopsi, L., Larsson, L.I. (1985) Increased sensitivity in immunocytochemistry. Effects of double application of antibodies and of silver intensification on immunogold and peroxidase-antiperoxidase staining techniques. Histochemistry 82, 321–329
Skene, D.S., Browning, G., Jones, H.G. (1987) Model systems for the immunolocalization of cis, trans abscisic acid in plant tissues. Planta 172, 192–199
Sossountzov, L., Sotta, B., Maldiney, R., Sabbagh, I., Miginiac, E. (1986) Immunoelectron-microscopy localization of abscisic acid with colloidal gold on Lowicryl-embedded tissues of Chenopodium polyspermum L. Planta 168, 471–481
Sotta, B., Pilate, G., Pelèse, F., Sabbagh, I., Bonnet, M., Maldiney, R. (1987) An avidin-biotin solid phase ELISA for femtomole isopentenyladenine and isopentenyladenosine measurements in HPLC purified plant extracts. Plant Physiol. 84, 571–573
Sotta, B., Sossountzov, L., Maldiney, R., Sabbagh, I., Tachon, P., Miginia, E. (1985) Abscisic acid localization by light microscopic immunohistochemistry in Chenopodium polyspermum L. Effect of water stress. J. Histochem. Cytochem. 33, 201–208
Spurr, A.R., (1969) A low viscosity epoxy resin embedding medium for electronmicroscopy. J. Ultrastruct. Res. 26, 31–43
Sternberger, L.A., Hardy, P.H., Jr., Cuculis, J.J., Meyer, H.G. (1970) The unlabelled antibody enzyme method of immunohistochemistry. Preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-anti-horseradish peroxidase) and its use in identification of Spirochetes. J. Histochem. Cytochem. 18, 315–333
Swaab, D.F. (1982) Comments on the validity of immunocytochemical methods. In: Cytochemical methods in neuroanatomy, pp. 423–440, Palay, S.L., Chan-Palay, V., eds. A.R. Liss, New York
Tucker, D.J. (1976) Endogenous growth regulators in relation to side shoot development in the tomato. New Phytol. 77, 561–568
Tucker, D.J. (1977) Hormonal regulation of lateral bud growth in the tomato. Plant. Sci. Lett. 8, 105–111
Tucker, D.J. (1979) Apical dominance in the tomato: Some further observations on isogenic lines showing varying degrees of side-shoot development. Ann. Bot. 43, 571–577
Tucker, D.J. (1981) Axillary bud formation in two isogenic lines of tomato showing different degrees of apical dominance. Ann Bot. 48, 837–843
Van Leeuwen, F. (1986) Pitfalls in immunocytochemistry with special reference to the specificity problems in the localization of neuropeptides. Am. J. Anat. 175, 363–377
Weiler, E.W. (1980) Radioimmunoassays for trans-zeatin and related cytokinins. Planta 149, 155–162
Weiler, E.W. (1984) Immunoassay of plant regulators. Annu. Rev. Plant Physiol. 35, 85–95
Weiler, E.W., Ziegler, H. (1981) Determination of phytohormones in phloem exudate from tree species by radioimmunoassay. Planta 152, 168–170
Yensen, J. (1968) Removal of epoxy resin from histological sections following halogenation. Stain Technol. 43, 344–346
Zavala, M.E., Brandon, D.L. (1983) Localization of a phytohormone using immunocytochemistry. J. Cell Biol. 97, 1235–1239
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sossountzov, L., Maddiney, R., Sotta, B. et al. Immunocytochemical localization of cytokinins in Craigella tomato and a sideshootless mutant. Planta 175, 291–304 (1988). https://doi.org/10.1007/BF00396334
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00396334