Abstract
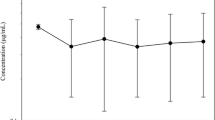
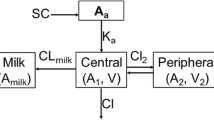
The pharmacokinetics of ceftazidime (CAZ) were studied in lactating (LTG) and non-lactating (NLTG) cows. Two groups (LTG and NLTG) of 5 healthy dairy cows were given ceftazidime (10 mg/ kg body weight) intravenously (i.v.) and intramuscularly (i.m.). Serum and milk (LTG) and serum samples (NLTG) were collected over a 24-h period post-administration. CAZ concentrations in serum and milk were determined by high-performance liquid chromatography, and an interactive and weighted-non-linear least-squares regression analysis was used to perform the pharmacokinetic analysis. The pharmacokinetic profiles in LTG and NLTG cows which had received CAZ i.v. fitted a three-compartment model and a two-compartment model, respectively. The CAZ concentration-time curves in serum and the area under the curve were greater and more sustained (p<0.05) in the LTG cows by both routes, while the serum clearance (Cls=72.5±18.1 ml/h per kg) was lower (p<0.05) than that in the NLTG cows (Cls=185.9±44.2 ml/h per kg). CAZ given i.v. exhibited a relatively long half-life of elimination (t 1/2 β (LTG)=1.1±0.2 h; t 1/2 β (NLTG)=1.4±0.3 h). Compared with other cephalosporins, CAZ had good penetration into the mammary gland (47.7±38.2% for CAZ i.v.; 51.1±39.0% for CAZ i.m.). Finally, the bioavailability of CAZ (F(LTG)=98.9±36.8%; F(NLTG)=77.1±25.3%) was suitable for its use by the i.m. route in lactating and non-lactating cows.
Similar content being viewed by others
Abbreviations
- AIC:
-
Akaike information criterion
- AUC:
-
area under the curve
- b.w.:
-
body weight
- CAZ:
-
ceftazidime
- Cls :
-
total serum clearance
- C max :
-
peak serum concentration
- COM:
-
compartment open model
- i.m.:
-
intramuscular(ly)
- i.v.:
-
intravenous(ly)
- LTG:
-
lactating
- K :
-
rate constant
- 1:
-
central compartment
- 2:
-
peripheral compartment
- 3:
-
deep compartment
- NLTG:
-
nonlactating
- t max :
-
time of peak serum concentration
- t 1/2 :
-
half-life
References
Akaike, A., 1978. Posterior probabilities for choosing a regression model. Annals of the Institute of Mathematical Statistics, 30, 9–14
Baggot, J.D., 1977. Principles of Pharmacokinetics, (W.B. Saunders, Philadelphia)
Balant, L., Dayer, P. and Auckenthaler, R., 1985. Clinical pharmacokinetics of the third generation cephalosporin. Clinical Pharmacokinetics, 10, 101–143
Barza, M., 1981. Principles of tissue penetration of antibiotics. Journal of Antimicrobial Chemotherapy, 8(supplement C), 7–28
Blanco, J.D., Jorgenssen, J.H., Castañeda, Y.S. and Crawford, S.A., 1983. Ceftazidime levels in human breast milk. Antimicrobial Agents and Chemotherapy, 23, 479–480
Capel, E.K. and Pratt, D.A.H., 1981. Renal tolerance of ceftazidime in animals. Journal of Antimicrobial Chemotherapy, 8(supplement B), 241–245
Gibaldi, M. and Perrier, D., 1982, Pharmacokinetics, 2nd edn, (Marcel Dekker, New York)
Harding, S.M., Munro, A.J., Thornton, J.E., Ayrton, J. and Hogg, M.I.J., 1981. The comparative pharmacokinetics of ceftazidime and cefotaxime in healthy volunteers. Journal of Antimicrobial Chemotherapy, 8(supplement B), 263–272
Jones, R.N., Barry, A.L., Thornsberry, C., Gerlach, E., Fuchs, P.C., Gavan, T.L. and Sommers, H.M., 1981. Ceftazidime, a pseudomonas-active cephalosporin: in vitro antimicrobial activity evaluation including recommendations for disc diffusion susceptibility test. Journal of Antimicrobial Chemotherapy, 8(supplement B), 187–211
Matsui, H., Komiya, M., Ikeda, C.H. and Tachibana, A., 1984. Comparative pharmacokinetics of YM-13115, ceftriaxone, and ceftazidime in rats, dogs, and rhesus monkeys. Antimicrobial Agents and Chemotherapy, 26, 204–207
Metzler, C.M. and Tong, D.D.M., 1981. Computational problems of compartmental models with Michaelis-Menten-type elimination. Journal of Pharmaceutical Science, 70, 733–737
O'Callaghan, C.H., Acred, P., Harper, P.B., Ryan, D.M., Kirby, S.M. and Harding, S.M., 1980. GR20263 a new broad-spectrum cephalosporin with antipseudomonal activity. Antimicrobial Agents and Chemotherapy, 17, 876–883
Rule, R., Rubio, M. and Perelli, M.C., 1991. Pharmacokinetics of ceftazidime in sheep and its penetration into tissue and peritoneal fluids. Research in Veterinary Science, 51, 233–238
Ryan, D.M., Mason, V. and Harding, S.M., 1981. The penetration of ceftazidime into extravascular fluid. Journal of Antimicrobial Chemotherapy, 8(supplement A), 283–288
Saito, A., 1983. Studies on absorption, distribution, metabolism and excretion of ceftazidime in Japan. Journal of Antimicrobial Chemotherapy, 12(supplement A), 225–262
Soback, S. and Ziv, G., 1989. Pharmacokinetics of ceftazidime given alone and in combination with probenecid to unweaned calves. American Journal of Veterinary Research, 50, 1566–1569
Thornsberry, C., 1985. Review of in vitro activity of third generation cephalosporins and other newer betalactam antibiotics against clinically important bacteria. American Journal of Medicine, 79(supplement 2A), 14–20
Walstad, R.A., Hellum, K.B., Blika, S., Dale, L.G., Fredriksen, T., Myhre, K.I. and Spencer, G.R., 1983. Pharmacokinetics and tissue penetration of ceftazidime: studies on lymph, aqueous humour, skin blister, cerebrospinal and pleural fluid. Journal of Antimicrobial Chemotherapy, 12(supplement A), 275–282
Wise, R., Armstrong, G.C., Brown, R.M. and Andrews, J.M., 1981. The pharmacokinetics and tissue penetration of ceftazidime and cefamandole in healthy volunteers. Journal of Antimicrobial Chemotherapy, 8(supplement B), 277–282
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rule, R., Quiroga, G.H., Rubio, M. et al. The pharmacokinetics of ceftazidime in lactating and non-lactating cows. Veterinary Research Communications 20, 543–550 (1996). https://doi.org/10.1007/BF00396297
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00396297