Abstract
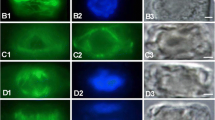
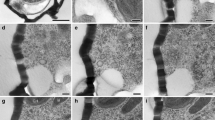
Microtubule (MT) arrangements were investigated, with immunofluorescence and electron microscopy, in two related species of coenocytic green algae. Intact cells of both Ernodesmis verticillata (Kützing) Boergesen and Boergesenia forbesii (Harvey) Feldmann have two morphologically distinct populations of MTs: a highly regular cortical array consisting of a single layer of parallel, longitudinal MTs; and perinuclear MTs radiating from the surface of the envelope of each interphase nucleus. In both algae, mitotic figures lack perinuclear MTs around them. Pre-incubation with taxol does not alter the appearance of these arrays. The cortical and nuclear MTs appear to coexist throughout the nuclear cycle, unlike the condition in most plant cells. At the cut/contracting ends of wounded Ernodesmis cells, cortical MTs exhibit bundling and marked convolution, with some curvature and slight bundling of MTs throughout the cell cortices. In Boergesenia, wound-induced reticulation and separation of the protoplasm into numerous spheres also involves a fasciation of MTs within the attenuating regions of the cytoplasm. Although some cortical MTs are fairly resistant to cold and amiprophos-methyl-induced depolymerization, the perinuclear ones are very labile, depolymerizing in 5–10 min in the cold. The MT cytoskeleton is not believed to be directly involved in wound-induced motility in these plants because amiprophos-methyl and cold depolymerize most cortical MTs without inhibiting motility. Also, the identical MT distributions in intact cells of these two algae belie the very different patterns of cytoplasmic motility. Although certain roles of the MT arrays may be ruled out, their exact functions in these plants are not known.
Similar content being viewed by others
Abbreviations
- APM:
-
amiprophos-methyl
- DIC:
-
differential interference contrast
- EGTA:
-
ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- FITC:
-
fluorescein isothiocyanate
- MT(s):
-
microtubule(s)
- PBS:
-
phosphate-buffered saline
References
Albertini, D.F., Clark, J.I. (1981) Visualization of assembled and disassembled microtubule protein by double label fluorescence microscopy. Cell Biol. Int. Rep. 5, 387–397
Clayton, L. (1985) The cytoskeleton and the plant cell cycle. In: The cell division cycle in plants, pp. 113–131, Bryan, J.A., Francis, D., eds. Cambridge University Press, Cambridge, UK
Cyr, R., Tochi, L., Fosket, D.E. (1984) Immunological studies on plant tubulins isolated from diverse cell lines. J. Cell Biol. 99, 41a
De Mey, J., Lambert, A.M., Bajer, A.S., Moeremans, M., De Brabander, M. (1982) Visualization of microtubules in interphase and mitotic plant cells of Haemanthus endosperm with the immuno-gold staining method. Proc. Natl. Acad. Sci. USA 79, 1898–1902
Derksen, J., Pierson, E.S., Traas, J.A. (1985) Microtubules in vegetative and generative cells of pollen tubes. Eur. J. Cell Biol. 38, 142–148
Doonan, J.H., Cove, D.J., Lloyd, C.W. (1985) Immunofluorescence microscopy of microtubules in intact cell lineages of the moss, Physcomitrella patens. I. Normal and CIPC-treated tip cells. J. Cell Sci. 75, 131–147
Enomoto, S., Hirose, H. (1972) Culture studies on artificially induced aplanospores and their development in the marine alga Boergesenia forbesii (Harvey) Feldmann (Chlorophyeae, Siphonocladales). Phycologia 11, 119–122
Franke, W.W., Seib, E., Osborn, M., Weber, K., Herth, W., Falk, H. (1977) Tubulin-containing structures in the anastral mitotic apparatus of endosperm cells of the plant Leucojum aestivum as revealed by immunofluorescence microscopy. Cytobiologie 15, 24–48
Garland, D.L. (1978) Kinetics and mechanism of colchicine binding to tubulin: evidence for ligand-induced conformational change. Biochemistry 17, 4266–4272
Gunning, B.E.S., Hardham, A.R. (1982) Microtubules. Annu. Rev. Plant Physiol. 33, 651–698
Hancock, K., Tsang, V.C.W. (1983) India ink staining of proteins on nitrocellulose paper. Anal. Biochem. 133, 157–162
Hayat, M.A. (1975) Fositive staining for electron microscopy. Van Nostrand Reinhold Co., New York
Hepler, P.K. (1985) The plant cytoskeleton. In: Botanical microscopy 1985, pp. 233–262, Robards, A.W., ed. Oxford University Press, Oxford, UK
Hori, T., Enomoto, S. (1978) Electron microscope observations on the nuclear division in Valonia ventricosa (Chlorophyceae, Siphonocladales). Phycologia 17, 133–142
Itoh, T., Brown, R.M., Jr. (1984) The assembly of cellulose microfibrils in Valonia macrophysa Kütz. Planta 160, 372–381
Jensen, W.A. (1962) Botanical histochemistry. Principles and practice. W.H. Freeman and Co., San Francisco
Johnson, G.D., Araujo, G.M.N. (1981) A simple method of reducing the fading of immunofluorescence during microscopy. J. Immunol. Meth. 43, 349–350
Kyhse-Andersen, J. (1984) Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J. Biochem. Biophys. Meth. 10, 203–209
La Claire, J.W., II. (1982) Cytomorphological aspects of wound healing in selected Siphonocladales (Chlorophyceae). J. Phycol. 18, 379–382
La Claire, J.W., II. (1984) Cell motility during wound healing in giant algal cells: contraction in detergent-permeabilized cell models of Ernodesmis. Eur. J. Cell Biol. 33, 180–189
Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685
Lloyd, C.W. (1984) Toward a dynamic helical model for the influence of microtubules on wall patterns in plants. Int. Rev. Cytol. 86, 1–51
Lloyd, C.W., Barlow, P.W. (1982) The co-ordination of cell division and elongation: the role of the cytoskeleton. In: The cytoskeleton in plant growth and development, pp. 203–228, Lloyd, C.W., ed. Academic Press, London
Lloyd, C.W., Clayton, L., Dawson, P.J., Doonan, J.H., Hulme, J.S., Roberts, I.N., Wells, B. (1985) The cytoskeleton underlying side walls and cross walls in plants: molecules and macromolecular assemblies. J. Cell Sci. Suppl. 2, 143–155
Lloyd, C.W., Seagull, R.W. (1985) A new spring for plant cell biology: microtubules as dynamic helices. Trends Biochem. Sci. 10, 476–478
Lloyd, C.W., Slabas, A.R., Powell, A.J., Lowe, S.B. (1980) Microtubules, protoplasts and plant cell shape. An immunofluorescent study. Planta 147, 500–506
Lloyd, C.W., Wells, B. (1985) Microtubules are at the tips of root hairs and form helical patterns corresponding to inner wall fibrils. J. Cell Sci. 75, 225–238
Mazia, D., Schatten, G., Sale, W. (1975) Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J. Cell Biol. 66, 198–200
Menzel, D. (1986) Visualization of cytoskeletal changes through the life cycle in Acetabularia. Protoplasma 134, 30–42
Menzel, D., Schliwa, M. (1986a) Motility in the siphonous green alga Bryopsis. I. Spatial organization of the cytoskeleton and organelle movements. Eur. J. Cell Biol. 40, 275–285
Menzel, D., Schliwa, M. (1986b) Motility in the siphonous green alga Bryopsis. II. Chloroplast movement requires organized arrays of both microtubules and actin filaments. Eur. J. Cell Biol. 40, 286–295
Mizuta, S., Wada, S. (1981) Microfibrillar structure of growing cell wall in a coenocytic green alga, Boergesenia forbesii. Bot. Mag. Tokyo 94, 343–353
Morejohn, L.C., Fosket, D.E. (1984) Inhibition of plant microtubule polymerization in vitro by the phosphoric amide herbicide amiprophos-methyl. Science 224, 874–876
Newcomb, E.H., Bonnett, H.T., Jr. (1965) Cytoplasmic microtubule and wall microfibril orientation in root hairs of radish. J. Cell Biol. 27, 575–589
Osborn, M., Webster, R.E., Weber, K. (1978) Individual microtubules viewed by immunofluorescence and electron microscopy in the same PtK2 cell. J. Cell Biol. 77, R27-R34
Schiff, P.B., Fant, J., Horwitz, S.B. (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277, 665–667
Staves, M.P., La Claire, J.W., II. (1985) Nuclear synchrony in Valonia macrophysa (Chlorophyta): light microscopy and flow cytometry. J. Phycol. 21, 68–71
Sternberger, L.A. (1986) Immunocytochemistry, 3rd edn. John Wiley & Sons, New York
Towbin, H., Staehelin, T., Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–4354
Traas, J.A., Braat, P., Emons, A.M.C., Meekes, H., Derksen, J. (1985) Microtubules in root hairs. J. Cell Sci. 76, 303–320
Van Lammeren, A.A.M., Keijzer, C.J., Willemse, M.T.M., Kieft, H. (1985) Structure and function of the microtubular cytoskeleton during pollen development in Gasteria verrucosa (Mill.) H. Duval. Planta 165, 1–11
Wang, K., Feramisco, J.R., Ash, J.F. (1982) Fluorescent localization of contractile proteins in tissue culture cells. Meth. Enzymol. 85, 514–562
Wick, S.M. (1985) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. III. Transition between mitotic/cytokinetic and interphase microtubule arrays. Cell Biol. Int. Rep. 9, 357–371
Wick, S.M., Duniec, J. (1983) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. I. Preprophase band development and concomitant appearance of nuclear envelope-associated tubulin. J. Cell Biol. 97, 235–243
Wick, S.M., Duniec, J. (1984) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. II. Transition between the pre-prophase band and the mitotic spindle. Protoplasma 122, 45–55
Woodcock, C.L. (1971) The anchoring of nuclei by cytoplasmic microtubules in Acetabularia. J. Cell Sci. 8, 611–621
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
La Claire, J.W. Microtubule cytoskeleton in intact and wounded coenocytic green algae. Planta 171, 30–42 (1987). https://doi.org/10.1007/BF00395065
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00395065