Abstract
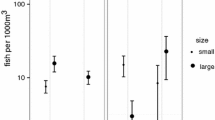
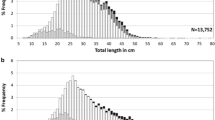
This paper contributes to the understanding of the evolution of alternative mating strategies by comparing morphological, ecological and ethological aspects of the reproductive systems of two closely related fish species studied near the marine biological station “STARESO” at Calvi, Corsica, between March 1982 and May 1985. The Mediterranean breeding areas of Tripterygion tripteronotus and T. delaisi partly overlap. The red territorial males of T. tripteronotus defend territories in the upper 6 m of the water column and the yellow territorial males of T. delaisi breed between a depth of 3 and 40 m. Small male T. tripteronotus possess relatively large gonads exhibit “sneaking” behaviour, while small male T. delaisi do not participate in mating. T. tripteronotus expends more effort in reproduction than T. delaisi. This is expressed in larger gonads, a higher density of and stronger competition for nest sites, and a longer breeding season and shorter spawning bouts with a higher fertilization rate in the former species. We hypothesize that the sneaking strategy of non-territorial male T. tripteronotus evolved in response to competition for nest sites in the shallow, upper water layers, which are limited in depth, but contain a good food supply. The depth restriction is imposed by the light-reflecting properties of the red territorial males. We suggest that the evolution of T. tripteronotus began with the appearance of a red morph of T. delaisi after the invasion of this latter species into the tideless Mediterranean Sea.
Similar content being viewed by others
Literature cited
Abel, E. F. (1955). Freilandbeobachtungen an Callionymus festivus Pall. und Tripterygion tripteronotus Risso, zwei Mittelmeerfishchen, unter besonderer Berücksichtigung des Fortpflanzungsverhaltens. Sber. öst. Akad. Wiss. (Math.-naturwiss. Kl. Abt. 1) 164 (10): 817–854
Coyne, J. A., Barton, N. H. (1988). What do we know about speciation? Nature, Lond. 331: 485–486
De Jonge, J., Videler, J. J. (unpublished a). Territorial versus sneaking reproductive strategies of Tripterygion tripteronotus males (Pisces, Teleostei)
De Jonge, J., De Ruiter, A. J. H., Van den Hurk, R. (unpublished b). Testis-testicular gland complex of two Tripterygion species (Blennioidei, Teleostei). Differences between territorial and non territorial males
Gordina, A. D., Duka, L. A., Oven, L. S. (1972). Sexual dimorphism, feeding and spawning in the ‘black-headed blenny’ (Tripterygion tripteronotus Risso) of the Black Sea. J. Ichthyol. (USSR) 12: 401–407
Gross, M. R. (1984). Sunfish, salmon and evolution of alternative reproduction strategies and tactics in fishes. In: Potts, G. W., Wootton, R. J. (eds.) Fish reproduction: strategies and tactics. Academic Press, London, p. 55–75
Gross, M. R., Charnov, E. L. (1980). Alternative male life histories in bluegill sunfish. Proc. natn. Acad. Sci. USA 77: 6937–6940
Robertson, D. R., Warner, R. R. (1978): Sexual patterns in the labroid fishes of the Western Caribbean. II: parrotfishes (Scaridae). Smithson. Contr. Zool. 255: 1–26
Warner, R. R., Lejeune, P. (1985). Sex change limited by parental care: a test using four Mediterranean labrid fishes, genus Symphodus. Mar. Biol. 87: 89–99
Warner, R. R., Robertson, D. R. (1978). Sexual patterns in the labroid fishes of the Western Caribbean. I: the wrasses. Smithson. Contr. Zool. 254: 1–27
Wirtz, P. (1977). Zum Verhalten blennioider Fische, insbesondere der mediterranen Tripteryglon Arten. Ph.D. thesis, Ludwig-Maximilian Universität, München
Wirtz, P. (1978). The behaviour of the Mediterranean Tripterygion species (Pisces, Blennioidei). Z. Tierpsychol. 48: 142–174
Wirtz, P. (1980). A revision of the Eastern-Atlantic Tripterygiidae (Pisces, Blennioidei) and notes on some westafrican blennioid fish. Cybium (3) 11: 83–101
Zander, C. D. (1973). Evolution of Blennioidei in the Mediterranean Sea. Revue Trav. Inst. (scient. tech.) Pech. marit. 37 (2): 215–221
Zander, C. D. (1982). Feeding ecology of littoral gobiid and blennioid fish of the Banyuls area (Mediterranean Sea). I: main food and trophic dimension of niche and ecotope. Vie Milieu 32 (1): 1–10
Zander, C. D., Heymer, A. (1970). Tripterygion tripteronotus (Risso, 1810) und Tripterygion xaenthosoma n. sp. Eine Ökologische Speziation (Pisces, Teleostei). Vie Milieu 21: 363–394
Author information
Authors and Affiliations
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
De Jonge, J., Videler, J.J. Differences between the reproductive biologies of Tripterygion tripteronotus and T. delaisi (Pisces, Perciformes, Tripterygiidae): the adaptive significance of an alternative mating strategy and a red instead of a yellow nuptial colour. Mar. Biol. 100, 431–437 (1989). https://doi.org/10.1007/BF00394818
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00394818