Abstract
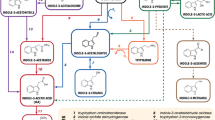
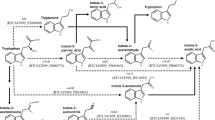
It has been proposed that the “eukaryotic” T-DNA-encoded indole-3-acetic acid (IAA) biosynthesis genes of Agrobacterium tumefaciens and their prokaryotic counterpart in Pseudomonas savastanoi originated from common ancestor genes. This paper provides additional evidence for the functional similarity between the gene products. We have demonstrated that a chimeric gene consisting of the coding sequence of the P. savastanoi tryptophan-2-mono-oxygenase (iaaM gene) and a plant promoter encodes an active enzyme in Nicotiana tabacum. Transformants obtained with this chimeric gene grew as a callus on hormone-free media. No stably transformed plantlets could be isolated. The callus tissues contained extremely high levels of indole-3-acetamide and slightly elevated levels of IAA. Either indole-3-acetamide by itself has a low auxin activity or, alternatively, it is converted aspecifically and at low rates into IAA. The P. savastanoi tryptophan-2-mono-oxygenase activity in plants is also able to detoxify the amino-acid analogue 5-methyltryptophan. This property can be used for positive selection of transformed calli.
Similar content being viewed by others
Abbreviations
- BAP:
-
6-benzylaminopurine
- IAA:
-
indole-3-acetic acid
- IAM:
-
indole-3-acetamide
- NAA:
-
naphthalene-1-acetic acid
- NPT-II:
-
neomycin phosphotransferase II
- T-DNA:
-
transferred DNA
References
Aerts, M., Jacobs, M., Hernalsteens, J.-P., Van Montagu, M., Schell, J. (1979) Induction and in vitro culture of Arabidopsis thaliana crown gall tumours. Plant Sci. Lett. 17, 43–50
Bolivar, F., Betlach, M., Heyneker, H.L., Shine, J., Rodriguez, R., Boyer, H.W. (1977) Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene 2, 75–93
Colson, C., Glover, S.W., Symonds, N., Stacey, K.A. (1965) The location of genes for host controlled modification and restriction in Escherichia coli. K 12. Genetics 52, 1043–1050
Comai, L., Kosuge, T. (1980) Involvement of plasmid deoxyribonucleic acid in indoleacetic acid synthesis in Pseudomonas savastanoi. J. Bacteriol. 143, 950–957
Comai, L., Kosuge, T. (1982) Cloning and characterization of iaaM, a virulence determinant of Pseudomonas savastanoi. J. Bacteriol. 149, 40–46
Dellaporta, S.L., Wood, J., Hicks, J.B. (1983) A plant DNA minipreparation: version II. Plant Mol. Biol. Reporter 1, 19–21
Dhaese, P., De Greve, H., Decraemer, H., Schell, J., Van Montagu, M. (1979) Rapid mapping of transposon insertion and deletion mutations in the large Ti-plasmids of Agrobacterium tumefaciens. Nucleic Acids Res. 7, 1837–1849
Follin, A., Inzé, D., Budar, F., Genetello, C., Van Montagu, M., Schell, J. (1985) Genetic evidence that the tryptophan 2-mono-oxygenase gene of Pseudomonas savastanoi is functionally equivalent to one of the T-DNA genes involved in plant tumour formation by Agrobacterium tumefaciens. Mol. Gen. Genet. 201, 178–185
Gheysen, G., Dhaese, P., Van Montagu, M., Schell, J. (1985) DNA flux across genetic barriers: the crown gall phenomenon. In: Advances in plant gene research, vol. 2: Genetic flux in plants, pp. 11–47, Hohn, B., Dennis, E.S., eds. Springer, Wien
Holsters, M., Silva, B., Van Vliet, F., Genetello, C., De Block, M., Dhaese, P., Depicker, A., Inzé, D., Engler, G., Villarroel, R., Van Montagu, M., Schell, J. (1980) The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid 3, 212–230
Inzé, D., Follin, A., Van Lijsebettens, M., Simoens, C., Genetello, C., Van Montagu, M., Schell, J. (1984) Genetic analysis of the individual T-DNA genes of Agrobacterium tumefaciens; further evidence that two genes are involved in indole-3-acetic acid synthesis. Mol. Gen. Genet. 194, 265–274
Inzé, D., Follin, A., Van Onckelen, H., Rüdelsheim, P., Schell, J., Van Montagu, M. (1987) Functional analysis of the T-DNA onc genes. In: Molecular biology of plant growth control (UCLA Symp. Mol. Biol. N. S., vol. 44, pp. 181–196, Fox, J.E., Jacobs, M., eds. Alan R. Liss, New York
Kanevsky, I.F., Gleba, Y.Y. (1986) Analysis of characters coded for by T-DNA using hybridization between tumorous and normal plant cells. Plant Cell Rep. 5, 352–355
Kosuge, T., Heskett, M.G., Wilson, E.E. (1966) Microbial synthesis and degradation of indole-3-acetic acid. 1. The conversion of L-tryptophan. J. Biol. Chem. 241, 3738–3744
Leemans, J., Shaw, C., Deblaere, R., De Greve, H., Hernalsteens, J.-P., Maes, M., Van Montagu, M., Schell, J. (1981) Site-specific mutagenesis of Agrobacterium Ti plasmids and transfer of genes to plant cells. J. Mol. Appl. Genet. 1, 149–164
Lemmers, M., De Beuckeleer, M., Holsters, M., Zambryski, P., Depicker, A., Hernalsteens, J.-P., Van Montagu, M., Schell, J. (1980) Internal organization, boundaries and integration of Ti-plasmid DNA in nopaline crown gall tumours. J. Mol. Biol. 144, 353–376
Linsmaier, E.M., Skoog, F. (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 18, 100–127
Maniatis, T., Fritsch, E.F., Sambrook, J. (1982) Molecular cloning, a laboratory manual, pp. 545. Cold Spring Harbor Laboratory, New York
Miller, J.H. (1972) Experiments in molecular genetics, pp. 466. Cold Spring Harbor Laboratory, New York
Otten, L.A., Schilperoort, R.A. (1978) A rapid microscale method for the detection of lysopine and nopaline dehydrogenase activities. Biochim. Biophys. Acta 527, 497–500
Reiss, B., Sprengel, R., Will, H., Schaller, H. (1984) A new sensitive method for qualitative and quantitative assay of neomycin phosphotransferase in crude cell extracts. Gene 30, 211–218
Sanger, M., Kosuge, T. (1984) Evidence of indoleacetamide is an intermediate in IAA biosynthesis of crown gall tumor tissue. Plant Physiol. Suppl. 75, 42
Schröder, G., Waffenschmidt, S., Weiler, E.W., Schröder, J. (1984) The T-region of Ti plasmids codes for an enzyme synthesizing indole-3-acetic acid. Eur. J. Biochem. 138, 387–391
Sembdner, G., Gross, D., Liebisch, H.-W., Schneider, G. (1980) Biosynthesis and metabolism of plant hormones. In: Encyclopedia of plant physiology, N.S., vol. 9: Hormonal regulation of development I, pp. 281–444, MacMillan, J., ed. Springer, Berlin Heidelberg New York
Simpson, J., Timko, M.P., Cashmore, A.R., Schell, J., Van Montagu, M., Herrera-Estrella, L. (1985) Light-inducible and tissue-specific expression of a chimaeric gene under control of the 5′-flanking sequence of a pea chlorophyll a/b-binding protein gene. EMBO J 4, 2723–2729
Thomashow, M.F., Hugly, S., Buchholz, W.G., Thomashow, L.S. (1986) Molecular basis for the auxin-independent phenotype of crown gall tumor tissues. Science 231, 616–618
Thomashow, L.S., Reeves, S., Thomashow, M.F. (1984) Crown gall oncogenesis: evidence that a T-DNA gene from the Agrobacterium Ti plasmid pTiA6 encodes an enzyme that catalyzes synthesis of indoleacetic acid. Proc. Natl. Acad. Sci. USA 81, 5071–5075
Van den Broeck, G., Timko, M.P., Kausch, A.P., Cashmore, A.R., Van Montagu, M., Herrera-Estrella, L. (1985) Targeting of a foreign protein to chloroplasts by fusion to the transit peptide of ribulose 1,5-bisphosphate carboxylase. Nature 313, 358–363
Van Lijsebettens, M., Inzé D., Van Montagu, M., Schell, J. (1986) Transformed cell clones as a tool to study T-DNA integration mediated by Agrobacterium tumefaciens. J. Mol. Biol. 188, 129–145
Van Onckelen, H., Prinsen, E., Inzé, D., Rüdelsheim, P., Van Lijsebettens, M., Follin, A., Schell, J., Van Montagu, M., De Greef, J. (1986) Agrobacterium T-DNA gene 1 codes for tryptophan 2-monooxygenase activity in tobacco crown gall cells. FEBS Lett. 198, 357–360
Van Onckelen, H., Rüdelsheim, P., Inzé D., Follin, A., Messens, E., Horemans, S., Schell, J., Van Montagu, M., De Greef, J. (1985) Tobacco plants transformed with the Agrobacterium T-DNA gene 1 contain high amounts of indole-3-acetamide. FEBS Lett. 181, 373–376
Velten, J., Schell, J. (1985) Selection-expression plasmid vectors for use in genetic transformation of higher plants. Nucleic Acids Res. 13, 6981–6998
Velten, J., Velten, L., Hain, R., Schell, J. (1984) Isolation of a dual plant promoter fragment from the Ti plasmid of Agrobacterium tumefaciens. EMBO J 3, 2723–2730
Vieira, J., Messing, J. (1982) The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19, 259–268
Willmitzer, L., Dhaese, P., Schreier, P.H., Schmalenbach, W., Van Montagu, M., Schell, J. (1983) Size, location, and polarity of T-DNA-encoded transcripts in nopaline crown gall tumors; evidence for common transcripts present in both octopine and nopaline tumors. Cell 32, 1045–1056
Yamada, T., Palm, C.J., Brooks, B., Kosuge, T. (1985) Nucleotide sequences of the Pseudomonas savastanoi indoleacetic acid genes show homology with Agrobacterium tumefaciens T-DNA. Proc. Natl. Acad. Sci. USA 82, 6522–6526
Zambryski, P., Joos, H., Genetello, C., Leemans, J., Van Montagu, M., Schell, J. (1983) Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J 2, 2143–2150
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Inzé, D., Follin, A., Velten, J. et al. The Pseudomonas savastanoi tryptophan-2-mono-oxygenase is biologically active in Nicotiana tabacum . Planta 172, 555–562 (1987). https://doi.org/10.1007/BF00393874
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00393874