Abstract
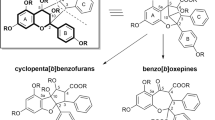
Clumps of white crystals present in 40-day-old malt agar cultures of Holwaya mucida were isolated as long white needles by crystallization from ethanol following short extraction with chloroform. The levorotary compound ([α] 21289 =-193.8°) was recognized as a γ-lactone (C17H20O5) by infrared and mass spectrometry. It was identified as 7α-methoxy-3a, 10b-dimethyl-1, 2, 3, 3aα, 5aα, 7, 10bβ, 10cα-octahydro-4H, 9H-furo[2′, 3′, 4′ : 4, 5]naphtho[2, 1-c]pyran-4, 9-dione, a labdane-derived compound known as antibiotic LL-Z1271α. Preparative thin-layer chromatography of the mother liquor afforded 2 minor metabolites. One was identified as LL-Z1271γ, the demethylated analogue of LL-Z1271α. The other one named LL-Z1271δ, was recognized as a compound related to α and γ: its structure could not be fully elucidated.
H. mucida (anamorph: Crinula calciiformis) has no taxonomic relationship with two other LL-Z1271α producing species viz. Acrostalagmus sp. (= Acremonium cf. atrogriseum) and Oidiodendron truncatum.
Similar content being viewed by others
References
Andersen, N.R., P.R. Rasmussen C.P. Falshaw & T.J. King (1984) The relative and absolute configuration of clerocidin and its cometabolites. Tetrahedron Lett. 25: 469–472
Ellestad, G.A., R.H. Evans Jr., M.P. Kunstmann, J.E. Lancaster & G.O. Morton (1970 Structure and chemistry of antibiotic LL-Z1271α, an antifungal carbon-17 terpene. J. Amer. Chem. Soc. 92: 5483–5489
Ellestad, G.A., R.H. Evans Jr. & M.P. Kunstmann (1971) LL-Z1271β, an additional C16 terpenoid metabolite from an Acrostalagmus species. Tetrahedron. Lett.: 497–500
Hughes, S.J. (1958) Revisiones Hyphomysetum aliquot. Can. J. Bot. 36: 727–836
Kakisawa, H., M. Sato, T. Ruo & T. Hayashi (1973) Biosynthesis of a C16-terpenoid lactone, a plant growth regulator. J. Chem. Soc. Chem. Comm.: 802–803
Korf, R.P. & G.S. Abawi (1971) On Holwaya, Crinula, Claussenomyces, and Corynella. Can. J. Bot. 49: 1879–1883
Krieglsteiner, G.J. & J. Häffner (1985) über Holwaya mucida (S. Schulzer von Müggenburg 1860) R.P. Korf et G.S. Abawi 1971, subsp. mucida Korf et Abawi 1971 und ihr Vorkommen in Europa. Z. Mycol. 51: 131–138
Sato, M. & H. Kakisawa (1976) Structures of three new C16 terpenoids from an Acrostalagmus fungus. J. Chem. Soc. Perkin Trans. I: 2407–2413
Seifert, K. (1985) A monograph of Stilbella and some allied Hyphomycetes. Stud. Mycol. 27: 1–235
Turner, W.B. (1971) Fungal Metabolites. Academic Press, London
Turner, W.B. & D.C. Aldridge (1983) Fungal Metabolites II. Academic Press, London
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Eijk, G.W., Roeijmans, H.J. & van der Aa, H.A. Labdane diterpene derivatives from Holwaya mucida . Antonie van Leeuwenhoek 54, 325–330 (1988). https://doi.org/10.1007/BF00393523
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00393523