Abstract
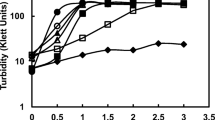
The marine cadmium-sensitive Acinetobacter CS 13 was grown in glucose-limited chemostats to study the chronic toxicity of CdCl2. Addition of 25, 50 or 100 μg Cd2+ l-1 caused disturbances of balanced growth. At the dilution rate (D) of 0.05 h-1 the washout rate (A) became constant at 84, 78 or 66 h after addition of 25, 50 or 100 μg Cd2+ l-1 The washout rates were calculated to be respectively 0.037 h-1, 0.039 h-1 and 0.077 h-1. At D=0.1 h-1, A became constant after 48, 42 and 36 h, and were the same as D for all three Cd concentrations. Acridine orange staining revealed that 99% of the cells fluoresced green (viable cells) and 1% red or orange (dead cells). However, under Cd stress the percentage of red and orange fluorescing cells increased up to 20%. At D=0.1 h-1, viable and direct cell counts always gave the same result. At D=0.05 h-1, however, viable cell counts were lower than direct cell counts after 2–3 d of Cd exposure. It is assumed that at D=0.05 h-1, a part of the cells appearing green was already Cd injured, but the true yellowish green colour was not identified. Decreasing cell densities were accompanied by increasing glucose concentrations. Cells accumulated Cd (0–25 μg Cd2+ l-1) in relation to exposure time and Cd concentration in the medium, but maximum Cd contents were the same, increasing from 5.5 or 5.7 μg g-1 dry wt in the control to 15 μg g-1 after 2–3 d (D=0.05 h-1) or 16 μg g-1 after 1–2 d (D=0.1 h-1).
Similar content being viewed by others
Literature cited
Aiking, H., K. Kok, H. van Heerikhuizen and J. van't Riet: Adaptation to cadmium by Klebsiella aerogenes growing in continuous culture proceeds mainly via formation of cadmium sulfide. Appl. environ. Microbiol. 44, 938–944 (1982)
Babich, H. and C. Stotzky: Effects of oadmium on the biota: influence of environmental factors. In: Advances in applied microbiology, Vol. 23, pp 55–117. Ed. by D. Perlman, New York: Academic Press 1978
Blundell, M. R. and D. G. Wild: Inhibition of bacterial growth by metal salts. A survey of effects on the synthesis of ribonucleic acid and protein. Biochem. J. 115, 207–212 (1969)
Chopra, I.: Mechanism of plasmid-mediated resistance to cadmium in Staphylococcus aureus. Antimicrob. Agents Chemother. 7, 8–14 (1975)
Doyle, J. J., R. T. Marshall and W. H. Pfander: Effects of cadmium on the growth and uptake of cadmium by microorganisms. Appl. Microbiol. 29, 562–564 (1975)
Flick, D. F., H. F. Kraybill and J. M. Dimitroff: Toxic effects of cadmium: a review. Environ. Res. 4, 71–85 (1971)
Fukushima, M.: Environmental pollution by cadmium and its health effects: an epidemiological approach to the “Itai-Itai” disease. In: New methods in environmental chemistry and toxicology, pp 231–252. Ed. by F. Coulston, F. Korte and M. Goto. Tokyo: International Academic Printing 1973
Gadd, G. M. and A. J. Griffiths: Microorganisms and heavy metal toxicity. Microb. Ecol. 4, 303–317 (1978)
Gauthier, M. J. and G. N. Flatau: Étude de l' accumulation du cadmium par une bactérie marine en fonction des conditions de cultures. Chemosphere 9, 713–718 (1980)
Groves, D. J., G. A. Wilson and F. E. Young: Inhibition of transformation of Bacillus subtilis by heavy metals. J. Bacteriol. 120, 219–226 (1974)
Hobbie, J. E., R. J. Daley and S. Jaspers: Use of nucleopore filters for counting bacteria by fluorescence microscopy. Appl. environ. Microbiol. 33, 1225–1228 (1977)
Jannasch, H. W.: Growth kinetics of aquatic bacteria. In: Aquatic microbiology, pp 55–68. Ed. by F. A. Skinner and J. M. Shewan. New York: Academic Press 1977
Kurek, E., J. Czaban and J.-M. Bollag: Sorption of cadmium by microorganisms in competition with other soil constituents. Appl. environ. Microbiol. 43, 1011–1015 (1982)
Mayfield, C. I., W. E. Inniss and P. Sain: Continuous culture of mixed sediment bacteria in the presence of mercury. Water Air Soil Pollut. 13, 335–349 (1980)
Mitra, R. S., R. H. Gray, B. Chin and I. A. Bernstein: Molecular mechanisms of accommodation in Escherichia coli to toxic levels of Cd2+. J. Bacteriol. 121, 1180–1188 (1975)
Mitra, R. S. and I. A. Bernstein: Nature of the repair process associated with the recovery of Escherichia coli after exposure to Cd2+. Biochem. biophys. Res. Commun. 74, 1450–1455 (1977)
Mitra, R. S. and I. A. Bernstein: Single-strand breakage in DNA of Escherichia coli exposed to Cd2+. J. Bacteriol. 133, 75–80 (1978)
Novick, R. P. and C. Roth: Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J. Bacteriol. 95, 1335–1342 (1968)
Pickett, A. W. and A. C. R. Dean: Cadmium and zinc sensitivity and tolerance in Klebsiella (Aerobacter) aerogenes. Microbios 15, 79–91 (1976)
Ramamoorthy, R. and D. J. Kushner: Binding of mercuric and other heavy metal ions by microbial growth media. Microb. Ecol. 2, 162–176 (1975)
Rigler, R., Jr.: Microfluorometric characterization of intracellular nucleic acids and nucleoproteins by acridine orange. Acta Physiol. Scand. 67 (Suppl. 267), 1–122 (1966)
Seiler, H.: Taxonomische Untersuchungen über Acinetobacter aus Oberflächenwasser. Arch. Hydrobiol. 85, 57–71 (1979)
Simpson, W. R.: A critical review of cadmium in the marine environment. Prog. Oceanogr. 10, 1–70 (1981)
Sterritt, R. M. and J. N. Lester: Influence of bacterial growth on the forms of cadmium in defined culture media. Bull. Environ. Contam. Toxicol. 24, 196–203 (1980)
Strugger, S.: Fluoreszenzmikroskopie und Mikrobiologie, 194 pp. Hannover: Verlag M. & H. Schaper 1949
Tan, T. L.: Bestimmung von Blei und Cadmium in Meerwasser und marinen Sedimenten. Erfahrungen mit Chelex-100, dem PTFE-Autoklav und der flammenlosen Atomabsorption. Veröff. Inst. Meeresforsch. Bremerh. 16, 11–30 (1976)
Tan, T. L.: Effect of long-term lead exposure on the seawater and sediment bacteria from heterogeneous continuous flow cultures. Microb. Ecol. 5, 295–311 (1980)
Thormann, D.: Über die Wirkung von Cadmium und Blei auf die natürliche heterotrophe Bakterienflora im Brackwasser des Weser-Ästuars. Veröff. Int. Meeresforsch. Bremerh. 15, 237–267 (1975)
Thormann, D.: Versuche mit Bakterien des Weser-Ästuars zur Toxizität und Anreicherung von Blei und Cadmium. Diss. Univ. Braunschweig, 123 pp. (1977)
Thormann, D. and H. Weyland: Beziehungen zwischen verschiedenen Brackwasser- und Meeresbakterien und der wachstumshemmenden Wirkung von Cadmium und Blei. Veröff. Inst. Meeresforsch. Bremerh. 17, 163–188 (1979)
Titus, J. A. and R. M. Pfister: Effects of pH, temperature, and Eh on the uptake of cadmium by bacteria and an artificial sediment. Bull. Environ. Contam. Toxicol. 28, 697–704 (1982)
Tynecka, Z., Z. Gos and J. Zajac: Reduced cadmium transport determined by a resistance plasmid in Staphylococcus aureus. J. Bacteriol. 147, 304–312 (1981a)
Tynecka, Z., Z. Gos and J. Zajac: Energy-dependent efflux of cadmium coded by a plasmid resistance determinant in Staphylococcus aureus. J. Bacteriol. 147, 313–319 (1981b)
Author information
Authors and Affiliations
Additional information
Communicated by O. Kinne, Hamburg
Rights and permissions
About this article
Cite this article
Tan, T.L. Unbalanced growth of Acinetobacter CS 13 in glucose-limited chemostats caused by sublethal CdCl2 concentrations. Marine Biology 76, 247–252 (1983). https://doi.org/10.1007/BF00393024
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00393024