Summary
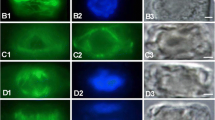
The subcellular distribution of actin was investigated in two related species of coenocytic green algae, with immunofluorescence microscopy. Either no, or fine punctate fluorescence was detected in intact cells of Ernodesmis verticillata (Kützing) Børgesen and Boergesenia forbesii (Harvey) Feldmann. A reticulate pattern of fluorescence appears throughout the cortical cytoplasm of Ernodesmis cells shortly after wounding; this silhouettes chloroplasts and small vacuoles. Slender, longitudinal bundles of actin become evident in contracting regions of the cell, superimposed over the reticulum. Thicker portions of the bundles were observed in well-contracted regions, and the highly-convoluted appearance of nearby cortical microtubules indicates contraction of the bundles in these thicker areas. Bundles are no longer evident after healing; only the reticulum remains. In Boergesenia, a wider-mesh reticulum of actin develops in the cortex of wounded cells, which widens further as contractions continue. Cells wounded in Ca2+-free medium do not contract, and although the actin reticulum is apparent, no actin bundles were ever observed in these cells. Exogenously applied cytochalasins have no effect on contractions of cut cells or extruded cytoplasm, and normal actin-bundle formation occurs in treated cells. In contrast, erythro-9-[3-(2-hydroxynonyl)]adenine (EHNA) completely inhibits longitudinal contractions in wounded cells, and few uniformly slender actin bundles develop in inhibited cells. These results indicate that wounding stimulates a Ca2+-dependent, hierarchical assembly of actin into bundles, whose assembly and functioning are inhibited by EHNA. Contraction of the bundles and concomitant wound healing are followed by cessation of motility and disassembly of the bundles. The spatial and temporal association of the bundles with regions of cytoplasmic contraction, indicates that the actin bundles are directly involved in wound-induced cytoplasmic motility in these algae.
Similar content being viewed by others
Abbreviations
- EHNA:
-
erythro-9-[3-(2-hydroxynonyl)]adenine
- MT(s):
-
microtubule(s)
References
Bestagno, M., Cerino, A., Riva, S., Ricotti, G.C.B.A. (1987) Improvements of western blotting to detect monoclonal antibodies. Biochem. Biophys. Res. Comm. 146, 1509–1514
Burgoyne, R.D., Cheek, T.R. (1987) Reorganisation of peripheral actin filaments as a prelude to exocytosis. Biosci. Rep. 7, 281–288
Cyr, R., Tochi, L., Fosket, D.E. (1984) Immunological studies on plant tubulins isolated from diverse cell lines. J. Cell Biol. 99, 41a
Dazy, A.-C., Hoursiangou-Neubrun, D., Sauron, M.-E. (1981) Evidence for actin in the marine alga. Acetabularia mediterranea. Biol. Cell 41, 235–238
DeRosier, D.J., Tilney, L.G. (1982) How actin filaments pack into bundles. Cold Spring Harbor Symp. Quant. Biol. 46, 525–540
Detmers, P.A., Carboni, J.M., Condeelis, J. (1985) Localization of actin in Chlamydomonas using antiactin and NBD-phallacidin. Cell Motil. 5, 415–430
Haupt, W., Wagner, G. (1984) Chloroplast movement. In: Membranes and sensory transduction, pp. 331–375, Colombetti, G., Lenci, F., eds. Plenum Press, New York
Hauri, H.-P., Bucher, K. (1986) Immunoblotting with monoclonal antibodies: importance of the blocking solution. Anal. Biochem. 159, 386–389
Hepler, P.K. (1985) The plant cytoskeleton. In: Botanical microscopy 1985, pp. 233–262, Robards, A.W., ed. Oxford University Press, Oxford, UK
Kamiya, N. (1981) Physical and chemical basis of cytoplasmic streaming. Annu. Rev. Plant Physiol. 32, 205–236
Kamiya, N. (1986) Cytoplasmic streaming in giant algal cells: a historical survey of experimental approaches. Bot. Mag. Tokyo 99, 441–467
Kreis, T.E., Birchmeier, W. (1980) Stress fiber sarcomeres of fibroblasts are contractile. Cell 22, 555–561
La Claire, J.W., II. (1982a) Cytomorphological aspects of wound healing in selected Siphonocladales (Chlorophyceae). J. Phycol. 18, 379–384
La Claire, J.W., II. (1982b) Wound-healing motility in the green alga Ernodesmis: calcium ions and metabolic energy are required. Planta 156, 466–474
La Claire, J.W., II. (1984a) Actin is present in a green alga that lacks cytoplasmic streaming. Protoplasma 120, 242–244
La Claire, J.W., II. (1984b) Cell motility during wound healing in giant algal cells: contraction in detergent-permeabilized cell models of Ernodesmis. Eur. J. Cell Biol. 33, 180–189
La Claire, J.W., II (1987) Microtubule cytoskeleton in intact and wounded coenocytic green algae. Planta 171, 30–42
Lessard, J.L., Carlton, D., Edelbrock, C. (1983) Characterization of monoclonal antibodies to muscle actins. Fed. Proc. 42, 2213
Lloyd, C.W. (1987) The plant cytoskeleton: the impact of fluorescence microscopy. Annu. Rev. Plant Physiol. 38, 119–139
Lloyd, C.W., Clayton, L., Dawson, P.J., Doonan, J.H., Hulme, J.S., Roberts, I.N., Wells, B. (1985) The cytoskeleton underlying side walls and cross walls in plants: molecules and macromolecular assemblies. J. Cell Sci. Suppl. 2, 143–155
Marano, F., Galleron, C., Minty, A.J., Montarras, D., Bornens, M. (1982) An actin-like protein and gene in the unicellular green alga Dunaliella. Cell Biol. Int. Rep. 6, 1085–1092
Marc, J., Gunning, B.E.S. (1986) Immunofluorescent localization of cytoskeletal tubulin and actin during spermatogenesis in Pteridium aquilinum (L.) Kuhn. Protoplasma 134, 163–177
McCurdy, D.W., Williamson, R.E. (1987) An actin-related protein inside pea chloroplasts. J. Cell Sci. 87, 449–456
Menzel, D., Schliwa, M. (1986) Motility in the siphonous green alga Bryopsis. II. Chloroplast movement requires organized arrays of both microtubules and actin filaments. Eur. J. Cell Biol. 40, 286–295
Merriam, R.W., Christensen, K. (1983) A contractile ring-like mechanism in wound healing and soluble factors affecting structural stability in the cortex of Xenopus eggs and oocytes. J. Embryol. Exp. Morphol. 75, 11–20
Metcalf, T.N., III, Szabo, L.J., Schubert, K.R., Wang, J.L. (1980) Immunochemical identification of an actin-like protein from soybean seedlings. Nature 285, 171–172
Niggli, V., Burger, M.M. (1987) Interaction of the cytoskeleton with the plasma membrane. J. Membr. Biol. 100, 97–121
Nothnagel, E.A., Sanger, J.W., Webb, W.W. (1982) Effects of exogenous proteins on cytoplasmic streaming in perfused Chara cells. J. Cell Biol. 93, 735–742
Parthasarathy, M.V. (1987) In situ localization of actin filaments in higher plant cells using fluorescent probes. Plant Mol. Biol. Rep. 5, 251–259
Pollard, T.D., Cooper, J.A. (1986) Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu. Rev. Biochem. 55, 987–1035
Pollard, T.D., Selden, S.C., Maupin, P. (1984) Interaction of actin filaments with microtubules. J. Cell Biol. 99, 33s-37s
Quader, H., Schnepf, E. (1986) The cytoskeleton of plant cells: structural and functional aspects. Ber. Dtsch. Bot. Ges. 99, 297–306
Schliwa, M., Ezzell, R.M., Euteneuer, U. (1984) erythro-9-[3-(2-hydroxynonyl)]adenine is an effective inhibitor of cell motility and actin assembly. Proc. Natl. Acad. Sci. USA 81, 6044–6048
Schmit, A.-C., Lambert, A.-M. (1987) Characterization and dynamics of cytoplasmic F-actin in higher plant endosperm cells during interphase, mitosis, and cytokinesis. J. Cell Biol. 105, 2157–2166
Schroeder, T.E. (1987) The origin and action of the contractile ring. In: Biomechanics of cell division (NATO ASI Ser. A, vol. 132), pp. 209–230, Akkas, N., ed. Plenum Press New York
Scagull, R.W., Falconer, M.M., Weerdenburg, C.A. (1987) Microfilaments: dynamic arrays in higher plant cells. J. Cell Biol. 104, 995–1004
Shimmen, T., Yano, M. (1986) Regulation of myosin sliding along Chara actin bundles by native skeletal muscle tropomyosin. Protoplasma 132, 129–136
Studier, F.W. (1973) Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J. Mol. Biol. 79, 237–248
Taylor, D.L., Wang, Y.-L., Heiple, J.M. (1980) Contractile basis of ameboid movement. VII. The distribution of fluorescently labeled actin in living amebas. J. Cell Biol 86, 590–598
Tiezzi, A., Moscatelli, A., Milanesi, C., Ciampolini, F., Cresti, M. (1987) Taxol-induced structures derived from cytoskeletal elements of the Nicotiana pollen tube. J. Cell Sci. 88, 657–661
Towbin, H., Staehelin, T., Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–4354
Traas, J., Doonan, J.H., Rawlins, D.J., Shaw, P.J., Watts, J., Lloyd, C.W. (1987) An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associates with the dividing nucleus. J. Cell Biol. 105, 387–395
Varma, M., Aebi, U., Fleming, J., Leavitt, J. (1987) A 60-kDa polypeptide in mammalian cells with epitopes related to actin. Exp. Cell Res. 173, 163–173
Williamson, R.E. (1986) Organelle movements along actin filaments and microtubules. Plant Physiol. 82, 631–634
Williamson, R.E., McCurdy, D.W., Hurley, U.A., Perkin, J.L. (1987) Actin of Chara giant internodal cells. A single isoform in the subcortical filament bundles and a larger, immunologically related protein in the chloroplasts. Plant Physiol. 85, 268–272
Williamson, R.E., Perkin, J.L., McCurdy, D.W., Craig, S., Hurley, U.A. (1986) Production and use of monoclonal antibodies to study the cytoskeleton and other components of the cortical cytoplasm of Chara. Eur. J. Cell Biol. 41, 1–8
Witztum, A., Parthasarathy, M.V. (1985) Role of actin in chloroplast clustering and banding in leaves of Egeria, Elodea and Hydrilla. Eur. J. Cell Biol. 39, 21–26
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
La Claire, J.W. Actin cytoskeleton in intact and wounded coenocytic green algae. Planta 177, 47–57 (1989). https://doi.org/10.1007/BF00392153
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00392153