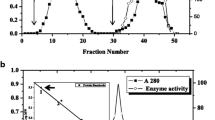
Abstract
Mobilization of the endosperm cell-wall reserves of Lactuca sativa L. cv. Grand Rapids requires endo-β-mannanase and α-galactosidase activity. A third enzyme, β-mannoside mannohydrolase (EC 3.2.1.25) is also involved. We have determined the optimum extraction and assay conditions for this enzyme, which is soluble only in high-salt (1 M NaCl) buffer. It is located exclusively within the cotyledons, in association with a cellulosic cell-wall fraction. Its substrates are the products of endosperm cell-wall mobilization, mannobiose and mannotriose, which diffuse to the cotyledons and are hydrolyzed extracellularly by the β-mannoside mannohydrolase.
Similar content being viewed by others
Abbreviations
- PNPM:
-
p-nitrophenyl-β-mannopyranoside
References
Bewley, J.D., Black, M. (1978) Physiology and biochemistry of seeds in relation to germination, vol. 1; Development, germination and growth. Springer-Verlag, Berlin Heidelberg New York
Bewley, J.D., Halmer, P. (1980/1981) Embryo-endosperm interaction in the hydrolysis of lettuce seed reserves. Israel J. Bot. 29, 118–132
Bewley, J.D., Leung, D.W.M., Ouellette, F.B. (1983) The cooperative role of endo-β-mannase, β-mannosidase and α-galactosidase in the mobilization of endosperm cell wall hemicelluloses of germinated lettuce seeds. In: Recent advances in phytochemistry, vol. 17: Mobilization of reserves in germination, pp. 137–152, Nozzolillo, C., Lea, P.J., Loewus, F.A., eds. Plenum Press, New York London
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., Smith, F. (1956) Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356
Eadie, G.S. (1942) The inhibition of cholinesterase by physostigmine and prostigmine. J. Biol. Chem. 146, 85–93
Evans, D.A., Brano, J.E. (1983) Protoplast isolation and culture. In: Handbook of plant cell culture, vol 1, pp. 124–176, Evans, D.A., Sharp, W.R., Ammirato, P.V., Yamato, Y., eds. McMillan Publishing Co., New York
Fersht, A. (1977) Enzyme structure and mechanism. W.H. Freeman and Co., San Francisco
Halmer, P., Bewley, J.D. (1982) Control by external and internal factors over the mobilization of reserve carbohydrates in higher plants. In: Encyclopedia of Plant Physiology, N.S., vol. 13A: Plant carbohydrates I, pp. 748–793, Loewus, F.A., Tanner, W., eds. Springer, Berlin Heidelberg New York
Halmer, P., Bewley, J.D., Thorpe, T.A. (1975) An enzyme to break down lettuce endosperm cell wall during gibberellin-and light-induced germination. Nature 258, 716–718
Hofstee, B.H.J. (1959) Non-inverted versus inverted plots in enzyme kinetics. Nature 184, 1296–1298
Huber, C.N., Scobell, H.D., Tai, H., Fisher, E.E. (1968) Thinlayer chromatography of the malto-oligo and megalosaccharides with mixed supports and multiple irrigations. Anal. Chem. 40, 207–209
Johnson, W.C., Lindsey, A.J. (1939) An improved universal buffer. Analyst 64, 490–492
Leung, D.W.M., Bewley, J.D. (1981) Immediate phytochrome action in inducing α-galactosidase activity in lettuce seeds. Nature 289, 587–588
Leung, D.W.M., Bewley, J.D. (1983) A role for α-galactosidase in the degradation of the endosperm cell wall in lettuce seed, cv. Grand Rapids. Planta 157, 274–277
Leung, D.W.M., Reid, J.S.G., Bewley, J.D. (1979) Degradation of the endosperm cell wall of Lactuca sativa L., cv. Grand Rapids in relation to mobilization of proteins and the production of hydrolytic enzymes in the axis, cotyledons and endosperm. Planta 146, 335–341
Loomis, W.D., Bataille, J. (1966) Plant phenolic compounds and the isolation of plant enzymes. Phytochemistry 5, 423–438
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, A.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275
McCleary, B.V. (1982) Purification and properties of a β-D-mannoside mannohydrolase from guar. Carbohydr. Res. 101, 75–92
McCleary, B.V. (1983a) β-D-Mannosidase from Helix pomatia. Carbohydr. Res. 111, 297–310
McCleary, B.V. (1983b) Enzymatic interaction in the hydrolysis of galactomannas in germinating guar: the role of exo-β-mannanase. Phytochemistry 22, 649–658
McCleary, B.V., Matheson, N.K. (1983) Action patterns and substrate binding requirements of β-mannanase with mannosacharides and mannan-type polysaccharides. Carbohyd. Res. 119, 191–219
McCleary, B.V., Matheson, N.K., Small, D.M. (1976) Galactomannans and galactoglucomannan in legume seed endosperms: structural requirements for β-mannanase hydrolysis. Phytochemistry 15, 1111–1117
McCleary, B.V., Taravel, R.F., Cheetham, N.W.H. (1982) Preparative-scale isolation and characterisation of 61-α-D-galactosyl-(1,4)-β-D-mannobiose and 62-α-D-galactosyl-(1,4)-β-D-mannobiose. Carbohydr. Res. 104, 284–297
McIlvaine, T.C. (1921) A buffer solution for colorimetric comparison. J. Biol. Chem. 49, 183–186
Meier, H., Reid, J.S.G. (1982) Reserve polysaccharides other than starch in higher plants. In: Encyclopedia of Plant Physiology, N.S., vol. 13A: Plant carbohydrates I, pp. 418–471, Loewus, F.A., Tanner, W., eds. Springer, Berlin Heidelberg New York
Powell, A.D., Leung, D.W.M., Bewley, J.D. (1983) Long-term storage of dormant Grand Rapids lettuce seeds in the imbibed state: physiological and metabolic changes. Planta 159, 182–188
Reese, E.T. (1977) Degradation of polymeric carbohydrates by microbial enzymes. In: Recent advances in phytochemistry, vol. 11: The structure, biosynthesis and degradation of wood, pp. 311–367, Loewus, F.A., Runeckles, V.C., eds. Plenum Press, New York London
Reese, E.T., Shibata, Y. (1965) β-Mannanase of fungi. Can. J. Microbiol. 11, 167–183
Scobes, R. (1982) Protein purification. Principle and practice. (Springer Advanced Texts in Chemistry, Cantor, C.R., Ser. ed.) Springer, New York Heidelberg Berlin
Sone, Y., Misaki, A. (1978) Purification and characterization of β-mannosidase and β-N-acetyl-D-hexosaminidase of Tremella fuciformis. J. Biochem. 83, 1135–1144
Woolf, L.I. (1953) Use of ion-exchange resins in paper chromatography of sugars. Nature 171, 841
Author information
Authors and Affiliations
Additional information
We dedicate this paper to the memory of our friend and colleague Dr. Jacob D. Duerksen
Rights and permissions
About this article
Cite this article
Ouellette, B.F.F., Bewley, J.D. β-Mannoside mannohydrolase and the mobilization of the endosperm cell wall of lettuce seeds, cv. Grand Rapids. Planta 169, 333–338 (1986). https://doi.org/10.1007/BF00392128
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00392128