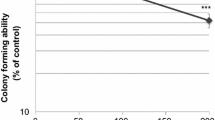
Summary
Nitrosodiphenylamine was tested for induction of DNA single strand breaks in rat hepatocytes and Chinese hamster V 79 cells with the alkaline filter elution assay. While in rat hepatocytes DNA damage was observed, negative results were obtained in V 79 cells. In view of the metabolic capacity of hepatocytes and the chemical structure of nitrosodiphenylamine it seems likely that cytochrome P-450-dependent, reductive denitrosation might be necessary for exerting this effect. Therefore the metabolism of nitrosodiphenylamine was investigated in phenobarbital-induced mouse liver microsomes and some of the metabolites were also tested. One metabolite was identified as diphenylamine whereas the others were identified as a ring-hydroxylated derivative of diphenylamine and its corresponding quinoneimine. Diphenylhydroxylamine which was not detected in the microsomes as a metabolite produced a significant amount of DNA single strand breaks in V 79 cells. When diphenylhydroxylamine was incubated with microsomes electron spin resonance spectrum was observed which indicated the formation of the diphenylnitroxide radical. This radical seems to be mediated by auto-oxidation rather than by enzymatic catalysis. Whether diphenylhydroxylamine might be responsible for the observed genetoxic effects of nitrosodiphenylamine assumed to be produced via active oxygen species is discussed.
Similar content being viewed by others
Abbreviations
- NDphA:
-
nitrosodiphenylamine
- DphA:
-
diphenylamine
- DphAOH:
-
diphenylhydroxylamine
- Pb:
-
phenobarbital
- Cyt.:
-
cytochrome
- DMSO:
-
dimethylsulphoxide
- MNNG:
-
N-methyl-N′-nitro-nitrosoguanidine
- NDBA:
-
N-nitroso-di-n-butylamine
- ESR:
-
electron spin resonance
- NO:
-
nitric oxide
References
Alexander WE, Ryan AJ, Wright SE (1965) Metabolism of diphenylamine in the rat, rabbit and man. Food Cosmet Toxicol 3:571–579
Appel KE, Graf H (1982) Metabolic nitrite formation from N-nitrosamines: evidence for a cytochrome P-450 dependent reaction. Carcinogenesis 3:293–296
Appel KE, Ruf HH, Mahr B, Schwarz M, Rickart R, Kunz W (1979) Binding of nitrosamines to cytochrome P-450 of liver microsomes. Chem Biol Interact 28:17–33
Appel KE, Schrenck D, Schwarz M, Mahr B, Kunz W (1980) Denitrosation of N-nitrosomorpholine by liver microsomes: possible role of cytochrome P-450. Cancer Lett 9:13–20
Appel KE, Rühl CS, Hildebrandt AG (1984a) Metabolic inactivation of N-nitrosamines by cytochrome P-450 in vitro and in vivo. In: O'Neill IK, von Borstel RC, Miller CT, Long J, Bartsch H (eds) N-Nitroso compounds: occurrence, biological effects and relevance to human cancer. IARC Scientific Publication No 57:443–451
Appel KE, Rühl CS, Spiegelhalder B, Hildebrandt AG (1984b) Denitrosation of diphenylnitrosamine in vivo. Toxicol Lett 23:353–358
Appel KE, Rühl CS, Hildebrandt AG (1985) Oxidative dealkylation and reductive denitrosation of nitrosomethylaniline in vitro. Chem Biol Interact 53:69–76
Appel KE, Wiessler M, Schoepke M, Rühl CS, Hildebrandt AG (1986a) Metabolic nitrite formation from N-nitrosamines: are there other pathways than reductive denitrosation by cytochrome P-450? Carcinogenesis 7:659–663
Appel KE, Görsdorf S, Scheper T, Bauszus M (1986 b) On the mechanism of action of diphenylnitrosamine. Naunyn-Schmiedeberg's Arch Pharmacol, Suppl 332:R 16
Argus MF, Hoch-Ligeti C (1961) Comparative study of the carcinogenic activity of nitrosamines. J Natl Cancer Inst 27:695–701
Boyland E, Carter RL, Gorrod JW, Roe FJC (1968) Carcinogenic properties of certain rubber additives. Eur J Cancer 4:233–239
Bradley MO, Dysart G, Fitzsimmons K, Harbach P, Lewin J, Wolf G (1982) Measurement by filter elution of DNA single and double strand breaks in rat hepatocytes: effects of nitrosamines and gamma irridation. Cancer Res 42:2592–2597
Bradley MO, Sina JF (1983) Methods for detecting carcinogens and mutagens with the alkaline elution rat hepatocytes assay. In: Kilbey BY, Leyator M, Nichols W, Ramel C (eds) Handbook of mutagenicity test procedures. Elvevier, North-Holland, pp 71–82
Cardy RH, Lijinsky W, Hildebrandt PK (1979) Neoplastic and non-neoplastic urinary bladder lesions induced in Fisher 344 rats and B6C3F1 hybrid mice by N-nitrosodiphenylamine. Ecotoxicol Environ Safety 3:29–35
Cesarone CF, Bolognesi C, Santi L (1979) Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Analytical Biochem 100:188–197
Druckrey H, Preussmann R, Ivankovic S, Schmähl D (1967) Organotrope carcinogene Wirkungen von 65 verschiedenen N-Nitroso-Verbindungen bei der BD-Ratte. Z Krebsforsch 69:103–201
Frederick CB, Weis CD, Flammang TJ, Martin CN, Kadlubar FF (1985) Hepatic N-oxidation, acetyltransfer and DNA binding of the acetylated metabolites of the carcinogen benzidine. Carcinogenesis 6:959–965
Iversen OH (1980) Tumorigenicity of N-nitroso-diethyl,-dimethyl, and-diphenyl amines in skin painting experiments. Eur J Cancer 16:695–698
Jones CA, Hubermann E (1980) A sensitive hepatocyte assay for the metabolism of nitrosamines to mutagens for mammalian cells. Cancer Res 40:406–411
Kaneko M, Nakayama T, Kodama M, Nagata Ch (1984) Detection of DNA lesions in cultured human fibroblasts induced by active oxygen species generated from a hydroxylated metabolite of 2-naphthyl-amine. Gann 75:349–354
Kanako M, Nagata Ch, Kodama M (1985) Repair of indirectly induced DNA damage in human skin fibroblasts treated with N-hydroxy-2-naphthylamine. Mutat Res 143:103–108
Kohn KW, Ewig RAG, Erickson LC, Zwelling LA (1981) Measurement of strand breaks and cross-links by alkaline elution. In: Friedberg ED, Hanawalt PC (eds) DNA repair. Dekker, New York, pp 379–401
Lai CC, Miller JA, Miller EC, Liem A (1985) N-sulfooxy-2-aminofluorene is the major ultimate electrophilic and carcinogenic metabolite of N-hydroxy-2-acetylaminofluorene in the livers of infant male C57BL/6J×C3H/HeJ F1 (B6C3F1) mice. Carcinogenesis 6:1037–1045
Mello Filho AC, Hoffmann ME, Meneghini R (1984) Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. Biochem J 218:273–275
Möller ME, Glowinski IB, Thorgeirsson SS (1984) The genotoxicity of aromatic amines in primary hepatocytes isolated from C57BL/6 and DBA/2 mice. Carcinogenesis 5:797–804
Nakayama T, Kimura T, Kodama M, Nagata Ch (1983) Generation of hydrogen peroxide and superoxide anion from active metabolites of naphthylamines and amino azo dyes: its possible role in carcinogenesis. Carcinogenesis 4:765–769
Schumann RF, Lebherz WB, Pienta RJ (1981) Induction of morphological transformation of hamster embryo cells in vitro by diphenylnitrosamine. Carcinogenesis 2:679–682
Stier A, Clauss R, Lücke A, Reitz I (1982) Radicals in carcinogenesis by aromatic amines. In: McBrien DCHM, Slater TF (eds) Free radicals, lipid peroxidation and cancer. Academic Press, New York, pp 329–351
Wakabayashi K, Nagao M, Kawachi T, Sugimura T (1981) Comutagenic effect of norharman with N-nitrosamine derivatives. Mutat Res 80:1–7
Wieland H, Roseeu A (1912) Zur Kenntnis des Diphenylhydroxylamins. Ber dt chem Ges 45:494–498
Wieland H, Offenbächer M (1914) Diphenylstickstoffoxyd, ein neues organisches Radikal mit vierwertigem Stickstoff. Ber dt chem Ges 47:2111–2115
Author information
Authors and Affiliations
Additional information
Dedicated to Professor Erich Hecker on the occasion of his 60th birthday
This work is part of the theses of S. Görsdorf and T. Scheper
Rights and permissions
About this article
Cite this article
Appel, K.E., Görsdorf, S., Scheper, T. et al. Metabolic denitrosation of diphenylnitrosamine: a possible bioactivation pathway. J Cancer Res Clin Oncol 113, 131–136 (1987). https://doi.org/10.1007/BF00391434
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00391434