Abstract
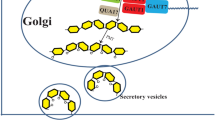
The pectic polysaccharides of spinach cell walls carry feruloyl groups on arabinose and galactose residues. The following experiments were designed to discover whether the arabinose residues are feruloylated intra-or extracellularly. Cultured spinach cells started to incorporate exogenous [3H]arabinose into polymers at a linear rate after a lag period of approx. 3–4 min, although radioactive polysaccharides and extensin did not start to appear outside the plasmalemma until after an approx. 25-min lag. In the same cells, polysaccharide-bound feruloyl-[3H]arabinose units starded to accumulate radioactivity at a linear rate after a lag period of approx. 4–5 min. Therefore, arabinose residues of polysaccharides began to be feruloylated while still intracellular. The rate of formation of polysaccharide-bound feruloyl-[3H]arabinose units did not appreciably increase after 25 min, showing that any additional extracellular feruloylation of the polysaccharide was relatively slow. This conclusion was supported by two different types of pulse-chase experiments, one of which was designed to detect feruloylation of polysaccharides up to 6 d after synthesis.
Similar content being viewed by others
Abbreviations
- Ara2 :
-
3-O−α-L-arabinopyranosyl-L-arabinose
- BAW:
-
butan-1-ol/acetic acid/water (12:3:5, by vol.)
- BEW:
-
butan-1-ol/ethanol/water (20:5:11, by vol.)
- EPW:
-
ethyl acetate/pyridine/water (8:2:1, by vol.)
- Fer-Ara2 :
-
3-O−(3-O−feruloyl-α-L-arabinopyranosyl)-L-arabinose
- Fer-Gal2 :
-
4-O−(6-O−feruloyl-β-D-galactopyranosyl)-D-galactose
References
Ahluwalia, B., Fry, S.C. (1986) Barley endosperm cell walls contain a feruloylated arabinoxylan and a non-feruloylated β-glucan. J. Cer. Sci. 4, 287–295
Fry, S.C. (1979) Phenolic components of the primary cell wall and their possible rôle in the hormonal regulation of growth. Planta 146, 343–351
Fry, S.C. (1982) Phenolic components of the primary cell wall: feruloylated disaccharides of D-galactose and L-arabinose from spinach polysaccharide. Biochem. J. 203, 493–504
Fry, S.C. (1983) Feruloylated pectins from the primary cell wall: their structure and possible functions. Planta 157, 111–123
Fry, S.C. (1984a) Isodityrosine—its detection, estimation and chemical synthesis. Methods Enzymol. 107, 388–397
Fry, S.C. (1984b) Incorporation of [14C]cinnamate into hydrolase-resistant components of the primary cell wall of spinach. Phytochemistry 23, 59–64
Fry, S.C. (1985) Primary cell wall metabolism. In: Oxford surveys of plant molecular and cell biology, vol. 2, pp. 1–42, ed. Miflin, B.J., Clarendon, Oxford
Fry, S.C. (1986a) Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annu. Rev. Plant Physiol. 37, 165–186
Fry, S. C. (1986b) In-vivo formation of xyloglucan nonasaccharide a possible biologically active cell-wall fragment. Planta 169, 443–453
Fry, S.C., Northcote, D.H. (1983) Sugar-nucleotide precursors of the arabinofuranosyl, arabinopyranosyl and xylopyranosyl residues of spinach polysaccharides. Plant Physiol. 73, 1055–1061
Geissmann, T., Neukom, H. (1971) Vernetzung von Phenolcarbonsäureestern von Polysacchariden durch oxydative phenolische Kupplung. Helv. Chim. Acta 54, 1108–1112
Harris, P.J., Hartley, R.D. (1980) Phenolic constituents of the cell walls of Monocotyledons. Biochem. Syst. Ecol. 8, 153–160
Hartley, R.D., Harris, P.J. (1981) Phenolic constituents of the cell walls of Dicotyledons. Biochem. Syst. Ecol. 9, 189–203
kolor, M.G., Roberts, H.R. (1957) A new reagent for the detection of hydroxyproline on paper chromatograms. Arch. Biochem. Biophys. 70, 620–622
Smith, J.J., Muldoon, E.P., Lamport, D.T.A. (1984) Isolation of extensin precursors by direct elution of intact tomato cell suspension cultures. Phytochemistry 23, 1233–1240
Tkotz, N., Strack, D (1980) Enzymatic synthesis of sinapoly-L-malate from 1-sinapoylglucose and L-malate by a protein preparation from Raphanus sativus cotyledons. Z. Naturforsch. 35c, 835–837
Yamamoto, E., Towers, G.H.N. (1985) Cell wall bound ferulic acid in barley seedlings during development and its photoisomerization. J. Plant Physiol. 117, 441–449
Zenk, M.H. (1979) Recent work on cinnamoyl CoA derivatives. Recent Adv. Phytochem. 12, 139–176
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fry, S.C. Intracellular feruloylation of pectic polysaccharides. Planta 171, 205–211 (1987). https://doi.org/10.1007/BF00391095
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00391095