Summary
The XP cell strain XP29MA, its malignant counterpart XP29MAmal and a normal human fibroblast strain were tested for colony-forming ability after treatment with HECNU in the presence of m6G, m6Gua, and he7G.
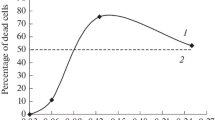
In XP29MAmal, inhibition of post-HECNU colony-forming ability was 35% when 0.25 mM of either m6G or m6Gua were present, whereas in XP29MA and the normal fibroblast strain no inhibition was detected. The he7G caused a similar but smaller inhibitory effect in XP29MAmal, but failed to do so in XP29MA.
HECNU predominantly exerts its killing effect by alkylating O-6 of DNA-bound guanine and causing DNA interstrand crosslinks. Alkylation of O-6 of guanine can be repaired by 6-methylguanine-DNA methyltransferase. From our experiments we conclude that in XP29MAmal this methyltransferase was inhibited in the presence of the 6-alkylguanines, thus leaving more 2-chloroethylated sites in DNA unrepaired. This results in sensitization in terms of decreased colony-forming ability observed only in the malignant cell line.
Similar content being viewed by others
Abbreviations
- XP:
-
xeroderma pigmentosum
- HECNU:
-
1-(2-chloroethyl)-1-nitroso-3-(2-hydroxyethyl)-urea
- HEPES:
-
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- m6G:
-
6-methylguanosine
- m6Gua:
-
6-methylguanine
- he7G:
-
7-(2-hydroxyethyl)-guanosine
References
Ashby J, Paton D, Styles JA, Greatbanks D, Wright B (1982) Synthesis of N7-hydroxyethylguanine and O6-hydroxyethylguanine. Mutat Res 103:257–261
Bedford P, Eisenbrand G (1984) DNA damage and repair in the bone marrow of rats treated with four chloroethylnitrosoureas. Cancer Res 44:514–518
Bogden JM, Eastman A, Bresnick E (1981) A system in mouse liver for the repair of O6-methylguanine lesions in methylated DNA. Nucleic Acids Res 9:3089–3103
Dolan ME, Kazushige M, Pegg AE (1985) Reduction of O6-alkylguanine-DNA alkyltransferase activity in HeLa cells treated with O6-alkylguanines. Cancer Res 45:6413–6417
Edler L, Thielmann HW (1986) Analysis of colony-forming ability of human fibroblast strains by linear regression. Biomed J (in press)
Edler L, Gröger P, Thielmann HW (1985a) Computational statistics for cell survival curves. I. Evaluation of the colony-forming ability of a single cell strain by the APL function CFALINE. Comput Programs Biomed 21:35–46
Edler L, Gröger P, Thielmann HW (1985b) Computational statistics for cell survival curves. II. Evaluation of colony-forming ability of a group of cell strains by the APL function CFAGROUP. Comput Programs Biomed 21:47–54
Eisenbrand G, Fiebig HH, Zeller WJ (1976) Some new congeners of the anticancer agent 1,3-bis(2-chloroethy)-1-nitrosourea (BCNU). Synthesis of bifunctional analogs and water soluble derivatives and preliminary evaluation of their chemotherapeutic potential. Z Krebsforsch 86:279–286
Eisenbrand G, Berger MR, Fiebig H, Müller N, Schreiber J, Stahl W, Sterzel W, Zeller WJ (1986) Drug design: nitrosoureas. IARC Sci Publ (in press)
Erickson LC, Bradley MO, Kohn KW (1978) Measurements of DNA damage in Chinese hamster cells treated with equitoxic and equimutagenic doses of nitrosoureas. Cancer Res 38:3379–3384
Huh N, Rajewsky MF (1986) Enzymatic elimination of O6-ethylguanine and stability of O4-ethylthymine in the DNA of malignant neural cell lines exposed to N-ethyl-N-nitrosourea in culture. Carcinogenesis 7:435–439
Lemaître M, Renard A, Verly WG (1982) A common chromatin factor involved in the repair of O6-methylguanine and O6-ethylguanine lesions in DNA. FEBS Lett 144:242–246
Mehta JR, Ludlum DB (1978) Synthesis and properties of O6-methyldeoxyguanylic acid and its copolymers with deoxycytidylic acid. Biochim Biophys Acta 521:770–778
Mehta JR, Ludlum DB, Renard A, Verly WG (1981) Repair of O6-ethylguanine in DNA by a chromatin fraction from rat liver: transfer of the ethyl group to an acceptor protein. Proc Natl Acad Sci USA 78:6766–6770
Myrnes B, Giercksky KE, Krokan H (1982) Repair of O6-methylguanine residues in DNA takes place by a similar mechanism in extracts from HeLa cells, human liver, and rat liver. J Cell Biochem 20:381–392
Myrnes B, Eggset G, Volden G, Krokan H (1984) Enzymatic repair of premutagenic DNA lesions in human epidermis. Quantitation of O6-methylguanine-DNA methyltransferase and uracil-DNA glycosylase activities. Mutat Res 131:183–186
Pegg AE, Wiest L (1983) Regulation of O6-methylguanine-DNA methyltransferase levels in rat liver and kidney. Cancer Res 43:972–975
Pegg AE, Roberfroid M, von Bahr C, Foote RS, Mitra S, Bresil H, Likhachev A, Montesano R (1982) Removal of O6-methylguanine from DNA by human liver fractions. Proc Natl Acad Sci USA 79:5162–5165
Pegg AE, Wiest L, Foote RS, Mitra S, Perry W (1983) Purification and properties of O6-methylguanine-DNA transmethylase from rat liver. J Biol Chem 258:2327–2333
Roe Jr R, Paul JS, Montgomery Jr PO'B (1973) Synthesis and PMR spectra of 7-hydroxyalkylguanosinium acetates. J Heterocycl Chem 10:849–857
Scudiero DA, Meyer SA, Clatterbuck BE, Mattern MR, Ziolkowski CH, Day III RS (1984) Sensitivity of human cell strains having different abilities to repair O6-methylguanine in DNA to inactivation by alkylating agents including chloroethylnitrosoureas. Cancer Res 44:2467–2474
Snedecor GW, Cochran WG (1967) Statistical Methods. Ames, University Press, Iowa State
Snow ET, Foote RS, Mitra S (1983) Replication and demethylation of O6-methylguanine in DNA. Prog Nucleic Acid Res Mol Biol 29:99–103
Thielmann HW, Popanda O, Edler L (1982) XP patients from Germany: correlation of colony-forming ability, unscheduled DNA synthesis and single-strand breaks after UV damage in xeroderma pigmentosum fibroblasts. J Cancer Res Clin Oncol 104:263–286
Thielmann HW, Fischer E, Dzarlieva RT, Komitowski D, Popanda O, Edler L (1983) Spontaneous in vitro malignant transformation in a xeroderma pigmentosum fibroblast line. Int J Cancer 31:687–700
Thielmann HW, Edler L, Popanda O, Friemel S (1985a) Xeroderma pigmentosum patients from the Federal Republic of Germany: decrease in post-UV colony-forming ability in 30 xeroderma pigmentosum fibroblast strains is quantitatively correlated with a decrease in DNA-incising capacity. J Cancer Res Clin Oncol 109:227–240
Thielmann HW, Hagedorn R, Freber W (1985b) Evaluation of colony-forming ability experiments using normal and DNA repair-deficient human fibroblast strains and an automatic colony counter. Cytometry 6:130–136
Thielmann HW, Edler L, Friemel S (1986) Xeroderma pigmentosum patients from Germany: repair capacity of 45 xeroderma pigmentosum fibroblast strains of the Mannheim XP Collection as measured by colony-forming ability and unscheduled DNA synthesis following treatment with methyl methanesulfonate and N-methyl-N-nitrosourea. J Cancer Res Clin Oncol 112:245–257
Waldstein EA, Cao E-H, Setlow RB (1982a) Adaptive resynthesis of O6-methylguanine-accepting protein can explain the differences between mammalian cells proficient and deficient in methyl excision repair. Proc Natl Acad Sci USA 79:5117–5121
Waldstein EA, Cao E-H, Miller ME, Cronkite EP, Setlow RB (1982b) Extracts of chronic lymphocytic leukemia lymphocytes have a high level of DNA repair activity of O6-methylguanine. Proc Natl Acad Sci USA 79:4786–4790
Yarosh DB (1985) The role of O6-methylguanine-DNA methyltransferase in cell survival, mutagenesis and carcinogenesis. Mutat Res 145:1–16
Yarosh DB, Fornace AJ, Day III RS (1985) Human O6-alkylguanine-DNA alkyltransferase fails to repair O4-methylthymine and methyl phosphotriesters in DNA as efficiently as does the alkyltransferase from Escherichia coli. Carcinogenesis 6:949–953
Yarosh DB, Hurst-Calderone S, Babich MA, Day III RS (1986) Inactivation of O6-methylguanine-DNA methyltransferase and sensitization of human tumor cells to killing by chloroethylnitrosourea by O6-methylguanine as a free base. Cancer Res 46:1663–1668
Zlotogorski C, Erickson LC (1983) Pretreatment of normal human fibroblasts and human colon carcinoma cells with MNNG allows chloroethylnitrosourea to produce DNA interstrand crosslinks not observed in cells treated with chloroethylnitrosourea alone. Carcinogenesis 4:759–763
Zlotogorski C, Erickson LC (1984) Pretreatment of human colon tumor cells with DNA methylating agents inhibits their ability to repair chloroethyl monoadducts. Carcinogenesis 5:83–87
Author information
Authors and Affiliations
Additional information
This work was supported by the Deutsche Forschungsgemeinschaft, SFB 136
The publication is dedicated to Professor E. Hecker on the occasion of his 60th birthday
Rights and permissions
About this article
Cite this article
Thielmann, H.W., Edler, L., Müller, N. et al. 6-Methylguanine and 6-methylguanosine inhibit colony-forming ability in a malignant xeroderma pigmentosum cell line but not in other xeroderma pigmentosum and normal human fibroblast strains after treatment with 1-(2-chloroethyl)-1-nitroso-3-(2-hydroxyethyl)-urea. J Cancer Res Clin Oncol 113, 67–72 (1987). https://doi.org/10.1007/BF00389969
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00389969