Summary
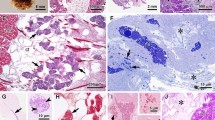
The salt gland in Spartina foliosa is composed of two cells, a large basal cell and a smaller, dome-shaped cap cell which is located on a neck-like protrusion of the basal cell. There is no cuticular layer separating the salt gland from the mesophyll tissue. The basal cell has dense cytoplasm which contains numerous mitochondria, rod-like wall protuberances, and infoldings of the plasmalemma which extend into the basal cell and partition the basal cell cytoplasm. The protuberances originate on the wall between the basal and the cap cells and are isolated from the basal-cell cytoplasm by the infoldings of the plasmalemma. While the cap cell has no partitioning membrane system or wall protuberances, it resembles the basal cell by having dense cytoplasm and numerous mitochondria.
The basal cell seems to be designed for efficient movement of ions toward the cap cell. The long, dead-end extracellular channels in the basal cell of Spartina appear comparable to surface specializations seen in the secreting epithelium of animal cells which carry out solute-linked water transport. The number of mitochondria and their close association with the plasmalemma extensions suggest that they have an important role in the transfer of ions through the basal cell.
The accumulated ions would move into the extracellular spaces along an osomotic gradient where the accompanying passive flow of water would move the ions into the cap-cell wall and from there the solution would pass out through the pores in the cuticle.
Similar content being viewed by others
References
Atkinson, M. R., Findlay, G. P., Hope, A. B., Pitman, M. G., Saddler, H. D. W., West, K. R.: Salt regulation in the mangroves Rhizophora mucronata Lam. and Aegialitis annulata R. Br. Aust. J. biol. Sci. 20, 589–599 (1967).
Berridge, M. J., Oschman, J. L.: A structural basis for fluid secretion by Malpighian tubules. Tissue and Cell 1, 247–272 (1969).
Bisalputra, T., Weier, T. E.: The cell wall of Scenedesmus quadricuada. Amer. J. Bot. 50, 1011–1019 (1963).
Copeland, D. E.: A study of salt secreting cells in the brine shrimp (Artemia salina). Protoplasma (Wien) 63, 363–384 (1967).
Diamond, J. M., Bossert, W. H.: Standing-gradient osmotic flow: a mechanism for coupling of water and solute transport in epithelia. J. gen. Physiol. 50, 2061–2084 (1967).
Ruhland, W.: Untersuchungen über die Hautdrüsen der Plumbaginaceen. Ein Beitrag zur Biologie der Halophyten. Jb. wiss. Bot. 55, 409–498 (1915).
Schmidt-Nielsen, B., Davis, L. E.: Fluid transport and tubular intercellular spaces in reptilian kidneys. Science 159, 1105–1108 (1968).
Schnepf, E.: Über Zellwandstrukturen bei den Köpfchendrüsen der Schuppenblätter von Lathraea clandestina L. Planta (Berl.) 60, 473–482 (1964).
Shimony, C., Fahn, A.: Light- and electron-microscopical studies on the structure of salt glands of Tamarix aphylla L. J. Linn. Soc. Bot. 60, 283–288 (1968).
Skelding, A. D., Winterbotham, J.: The structure and development of the hydathodes of Spartina Townsendii Groves. New Phytologist 38, 69–79 (1939).
Spurr, A. R.: A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26, 31–43 (1969).
Sutherland, G. H., Eastwood, A.: The physiological anatomy of Spartina Townsendii. Ann. Bot. 30, 333–351 (1916).
Thomson, W. W., Berry, W. L., Liu, L. L.: Localization and secretion of salt by the salt glands of Tamarix aphylla. Proc. nat. Acad. Sci. (Wash.) 63, 310–317 (1969).
—, Liu, L. L.: Ultrastructural features of the salt gland of Tamarix aphylla L. Planta (Berl.) 73, 201–220 (1967).
Venable, J. H., Coggeshall, R. J.: A simplified lead citrate stain for use in electron microscopy. J. Cell Biol. 25, 407–408 (1965).
Weier, T. E., Brown, D. L.: Formation of the prolamellar body in 8-day, darkgrown seedlings. Amer. J. Bot. 57, 267–275 (1970).
Ziegler, H., Lüttge, U.: Die Salzdrüsen von Limonium vulgare. I. [Mitteilung:] Die Feinstruktur. Planta (Berl.) 70, 193–206 (1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Levering, C.A., Thomson, W.W. The ultrastructure of the salt gland of Spartina foliosa . Planta 97, 183–196 (1971). https://doi.org/10.1007/BF00389200
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00389200