Summary
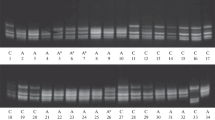
Changes in amylase isozyme patterns on polyacrylamide gels were followed during maturation in grains of Deba Abed barley. Early stage seeds contained a single, high-mobility enzyme (Band A) which had an estimated molecular weight of 4.2×104 and a high activity with β-limit dextrin as a substrate. It was shown, by dissection, that Band A was confined to the aleurone layer and probably represented the initial product of amylase synthesis.
This form was succeeded, in mid-course, by a less mobile form (Band B), a β-amylase with a molecular weight of approximately 1.3×105. Late-dough stage grains contained a complex of low-mobility β-amylase bands which were shown, by papain digestion, to be protein-bound forms of Band B.
The changes are discussed on the basis of a unified series consisting of elaborated forms of the initial Band A type of activity.
Similar content being viewed by others
References
Andrews, P.: Estimation of the molecular weights of proteins by sephadex gel filtration. Biochem. J. 91, 222–233 (1964).
Bernfeld, P.: Amylases α and β. Methods in enzymology, vol. 1 p. 149–158. New York: Academic Press 1955.
Coddington, A., Fincham, J. R. S., Sundaram, T. K.: Multiple active varieties of Neurospora glutamate dehydrogenase formed by hybridisation between two inactive mutant proteins in vivo and in vitro. J. molec. Biol. 17, 503–512 (1966).
Duffus, C. M.: α-amylase activity in the developing barley grain and its dependence upon gibberellic acid. Phytochem. 8, 1205–1209 (1969).
Engel, C.: The distribution of amylase, proteinase and esterase in resting cereals. Rec. Trav. chim. Pays-Bas 64, 318–320 (1945).
Englard, S., Singer, T. P.: Physicochemical studies on β-amylase. J. biol. Chem. 187, 213–220 (1950).
Evers, A. D.: Development of the endosperm of wheat. Ann. Bot., N.S. 34, 547–555 (1970).
Jacobsen, J. V., Scandalios, J. G., Varner, J. E.: Multiple forms of amylase induced by gibberellic acid in isolated barley aleurone layers. Plant Physiol. 45, 367–371 (1970).
Lowry, O. H., Rosebrough, N. J., Lewis-Farr, A., Randall, R. J.: Protein measurement with the folin phenol reagent. J. biol. Chem. 193, 265–275 (1951).
May, L. H., Buttrose, M. S.: Physiology of cereal grain. II. Starch granule formation in the developing barley kernel. Aust. J. biol. Sci. 12, 146–159 (1959).
Olered, R.: Development of α-amylase and falling number in wheat and rye during ripening. Thesis No 23. Agric. Coll. of Sweden, Uppsala. 1–101 (1967).
Paleg, L. G.: Physiological effects of gibberellic acid. 2. On starch hydrolysing enzymes of barley endosperm. Plant Physiol. 35, 902–906 (1960).
Price, G. M.: An apparatus for the electrophoresis of proteins on polyacrylamide gel. Lab. Pract. 17, 467–470 (1968).
Rowsell, E. V., Goad, L. J.: The constituent of wheat binding latent β-amylase. Biochem. J. 84, 73 P (1962).
Schwimmer, S., Balls, A. K.: Isolation and properties of crystalline α-amylase from germinated barley. J. biol. Chem. 179, 1063–1074 (1949).
Varner, J. E.: Gibberellic acid controlled synthesis of α-amylase in barley endosperm. Plant. Physiol. 39, 413–415 (1964).
Whelan, W. J.: Formation, mobilisation and transformation of carbohydrates. Starch and similar polysaccharides. Encyclop. of Plant Physiol., vol. VI, p. 154–240. Berlin-Göttingen-Heidelberg: Springer 1958.
Yomo, J.: Studies on the α-amylase activating substance. IV. On the amylase activating action of gibberellin. Hakko Kyokaishi. 18, 600–602 (1960).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stoddart, J.L. Sequential changes in amylase isozymes during grain maturation in barley. Planta 97, 70–82 (1971). https://doi.org/10.1007/BF00388407
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00388407