Summary
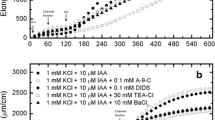
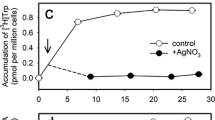
The enhancement by indoleacetic acid (IAA) of 36Cl- uptake into Avena coleoptile sections was used to study the effects of a hormone on a membrane-controlled phenomenon. Compared to sections in phosphate buffer only, Cl- content of the cells increases 15 min after addition of IAA; the promotion is seen only with growth-active auxins and is saturated at 3 μM IAA. The percent enhancement by IAA is the same over a wide range of Cl- concentrations. The hormone effect is not observed at ice-bath temperature and is not correlated with growth or water movement into the cells. IAA does not influence the movement of Cl- in the section. While auxin must be present within the tissue in order to maintain the enhancement, there is no relationship between the total amount of auxin and the accelerated Cl- uptake that results. A polarity in the auxin effect is implied since only apical applications of IAA promote Cl- uptake.
Similar content being viewed by others
References
Avery, G. S., Jr., Burkholder, P. R., Creighton, H. B.: Plant hormones and mineral nutrition. Proc. nat. Acad. Sci. (Wash.) 22, 673–678 (1936).
Blackman, G. E.: Interrelationships between the uptake of 2,4-dichlorophenoxyacetic acid, growth, and ion absorption. In: The chemistry and mode of action of plant growth substances, Wain, R. L., Wightman, F. W., eds., p. 253–259. New York: Acad. Press 1956.
Burström, H.: Studies on growth and metabolism of roots. IV. Positive and negative auxin effects on cell elongation. Physiol. Plantarum (Cph.) 3, 277–292 (1950).
Cleland, R., Bonner, J.: The residual effect of auxin on the cell wall. Plant Physiol. 31, 350–354 (1956).
Commoner, B., Mazia, D.: The mechanism of auxin action. Plant Physiol. 17, 682–685 (1942).
dela Fuente, R. K., Leopold, A. C.: Time course of auxin stimulations of growth. Plant Physiol. 46, 186–189 (1970).
Epstein, E.: Dual pattern of ion absorption by plant cells and by plants. Nature (Lond.) 212, 1324–1327 (1966).
Eshel, A., Waisel, Y.: Variations in sodium uptake along primary roots of corn seedlings. Plant Physiol. 49, 585–589 (1972).
Evans, M. L., Ray, P. M.: Timing of the auxin response in coleoptiles and its implications regarding auxin action. J. gen. Physiol. 53, 1–20 (1959).
Goldsmith, M. H.: The transport of auxin. Ann. Rev. Plant Physiol. 19, 347–360 (1968).
Goldsmith, M. H. M., Thimann, K. V.: Some characteristics of movement of indoleacetic acid in coleoptiles of Avena. I. Uptake, destruction, immobilization, and distribution of IAA during basipetal translocation. Plant Physiol. 37, 492–505 (1962).
Hertel, R., Evans, M. L., Leopold, A. C., Sell, H. M.: The specificity of the auxin transport system. Planta (Berl.) 85, 238–248 (1969).
Hertel, R., Leopold, A. C.: Versuche zur Analyse des Auxintransports in der Koleoptile von Zea mays L. Planta (Berl.) 59, 535–562 (1963).
Higinbotham, N., Etherton, B., Foster, R. J.: Mineral ion contents and cell transmembrane electropotentials of pea and oat seedling tissue. Plant Physiol. 42, 37–46 (1967).
Higinbotham, N., Graves, J. S., Davis, R. F.: Evidence for an electrogenic ion transport pump in cells of higher plants. J. Membrane Biol. 3, 210–222 (1970).
Higinbotham, N., Latimer, H., Eppley, R.: Stimulation of rubidium absorption by auxins. Science 118, 243–245 (1953).
Higinbotham, N., Pratt, M. J., Foster, R. J.: Effects of calcium, indoleacetic acid, and distance from stem apex on potassium and rubidium absorption by excised segments of etiolated pea epicotyl. Plant Physiol. 37, 203–214 (1962).
Ilan, I.: A specific stimulatory action of indolyl-3-acetic acid on potassium uptake by plant cells, with a concomitant inhibition of ammonium uptake. Nature (Lond.) 194, 203–204 (1962).
Ilan, I., Reinhold, L.: Analysis of the effects of indole-3-acetic acid on the uptake of monovalent cations. Physiol. Plantarum (Cph.) 16, 596–603 (1963).
Jönsson, A.: Chemical structure and growth activity of auxins and antiauxins. Encycl. Plant. Physiol., vol. XIV, p. 959–1006, Ruhland, W., ed. Berlin-Göttingen-Heidelberg: Springer 1961.
Jost, J.-P., Rickenberg, H. V.: Cyclic AMP. Ann. Rev. Biochem. 40, 741–774 (1971).
Köhler, D.: Über den Zusammenhang zwischen Achsen- und Blattwachstum und Ionenaufnahme bei Keimlingen von Pisum sativum. Z. Pflanzenphysiol. 63, 185–193 (1970).
Leopold, A. C.: Auxins and plant growth. Berkeley: Univ. of Calif. Press 1955.
Lüttge, U., Bauer, K., Köhler, D.: Frühwirkungen von Gibberellinsäure auf Membrantransport in jungen Erbsenpflanzen. Biochim. biophys. Acta (Amst.) 150, 452–459 (1968).
McRae, D. H., Bonner, J.: Chemical structure and antiauxin activity. Physiol. Plantarum (Cph.) 6, 485–510 (1953).
Nance, J. F.: Inhibition of salt accumulation in excised wheat roots by 2,4-dichlorophenoxyacetic acid. Science 109, 174–176 (1949).
Palmer, J. M.: The influence of growth regulating substances on the development of enhanced metabolic rates in thin slices of beet root storage tissue. Plant Physiol. 41, 1173–1178 (1966).
Pickles, V. R., Sutcliffe, J. F.: The effects of 5-hydroxytryptamine, indole-3-acetic acid, and some other substances on pigment effusion, sodium uptake, and potassium efflux by slices of red beet root in vitro. Biochim. biophys. Acta (Amst.) 17, 244–251 (1955).
Pierce, W., Higinbotham, N.: Compartments and fluxes of K, Na, and Cl in Avena coleoptile cells. Plant Physiol. 46, 666–673 (1970).
Riggs, T. R.: Hormones and transport across cell membranes. In: Biochemical actions of hormones, vol. I, p. 157–208, Litwack, G., ed. New York: Acad. Press 1970.
Scott, T. K., Briggs, W. R.: Auxin relationships in the Alaska pea (Pisum sativum). Amer. J. Bot. 47, 492–499 (1960).
Skoog, F.: Relationships between zinc and auxin in the growth of higher plants. Amer. J. Bot. 27, 939–951 (1940).
Smith, R.C., Epstein, R.: Ion absorption by shoot tissue: technique and first findings with excised leaf tissue of corn. Plant Physiol. 39, 338–341 (1964).
Spanswick, R. M., Williams, E. J.: Electrical potentials and Na, K, and Cl concentrations in the vacuole and cytoplasm of Nitella translucens. J. exp. Bot. 15, 193–200 (1964).
Steward, F. C., Mott, R. L.: Cells, solutes, and growth: salt accumulation in plants reexamined. Int. Rev. Cytol. 28, 275–370 (1970).
Went, F. W.: Transport of inorganic ions in polar plant tissues. Plant Physiol. 14, 365–369 (1939).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rubinstein, B., Light, E.N. Indoleacetic-acid-enhanced chloride uptake into coleoptile cells. Planta 110, 43–56 (1973). https://doi.org/10.1007/BF00386921
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00386921