Summary
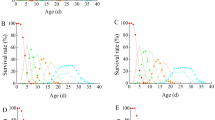
In vivo development of Echinostoma malayanum Leiper, 1911 was studied in white rats and the developmental process was arbitrarily divided into four stages: organogeny, vitellogenesis, formation of Mehlis' gland complex and cirrus sac, and oviposition. The percentage of development was 86–94. Population density affected the prepatent period of flukes and the normal prepatent period of 13–16 days was altered to 18–23 days in infection with 500–800 flukes. The majority of flukes in heavy infection were undersized and in the immature stage of development at patency. Data from chemical analysis of flukes revealed that protein, lipids, calcium and ash decreased quantitatively in flukes from higher population densities but no such change was observed as regards glycogen. Pathological changes in the rat intestine included lysis and destruction of mucosa, increased activity of goblet cells, oedematous and reticulated appearance of lamina propria and slight to moderate hyperplasia of epithelial cells. The metacercariae excysted in the medium containing trypsin plus sodium cholate and pepsin, though not essential for a high percentage of excystment, affected the rate. The reductant sodium dithionite substantially enhanced the rate and percentage of excystment. Excystation was optimal at pH 8, and 42° C was more effective than 39° C.
Similar content being viewed by others
References
Bell, E.J., Smyth, J.D.: Cytological and histochemical criteria for evaluating development of trematodes and pseudophyllidean cestodes in vivo and in vitro. Parasitology 48, 131–148 (1958)
Biester, H.E., Schwarte, L.H.: Diseases of poultry. Ames, Iowa, USA: Iowa State University Press 1965
Boray, J.C.: Experimental fascioliasis in Australia. Adv. Parasitol. 7, 95–210 (1969)
Brand, T. von: Biochemistry of parasites. New York, London: Academic Press 1966
Cable, R.M.: The resistance of herring gull Larus argentatus to experimental infections on the trematode Parorchis acanthus. J. Parasitol. 23, 559 (1937)
Ching, H.L.: The development and morphological variation of Philophthalmus gralli Mathis and Leger, 1910 with a comparison of species of Philophthalmus Looss, 1899. Proc. Helminth. Soc. Wash. 28, 130–138 (1961)
Clegg, J.A.: In vitro cultivation of Schistosoma mansoni. Exp. Parasitol. 16, 133–147 (1965)
Clegg, J.A., Smyth, J.D.: Growth, development and culture methods: Parasitic platyhelminths. Chem. Zool. 2, 395–466 (1968)
Colgan, G.J., Nollen, P.M.: Studies on growth, reproduction and infection of Philophthalmus hegeneri in the chick. In: Program and Abstracts, 51st Annual Meeting, Am. Soc. Parasitol., pp. 59–60 (1976)
Dawes, B.: On the growth and maturation of Fasciola hepatica L. in the mouse. J. Helminthol. 36, 11–38 (1962)
Dawes, B., Hughes, D.L.: Fascioliasis: the invasive stages of Fasciola hepatica in mammalian hosts. Adv. Parasitol. 2, 97–168 (1964)
Dixon, K.E.: The physiology of excystment of the metacercaria of Fasciola hepatica L. Parasitology 56, 431–456 (1966)
Erasmus, D.A.: The biology of trematodes. London: Edward Arnold Publishers Ltd. 1972
Erasmus, D.A., Bennett, L.J.: A study of some of the factors affecting excystation in vitro of the metacercarial stages of Holostephanus lühei Szidat, 1936 and Cyathocotyle bushiensis Khan, 1962 (Strigeida: Trematoda). J. Helminthol. 39, 185–196 (1965)
Foreyt, W.J., Todd, A.C.: Development of the large American liver fluke, Fascioloides magna in white tailed deer, cattle and sheep. J. Parasitol. 62, 26–32 (1976)
Fried, B.: Growth of Philophthalmus sp. (Trematoda) in the eyes of chicks. J. Parasitol. 48, 395–399 (1962)
Fried, B.: Infectivity, growth, development, excystation and transplantation of Zygocotyle lunata (Trematoda) in the chick. J. Parasitol. 56, 44–47 (1970)
Fried, B., Barber, L.W., Butler, M.S.: Infectivity, growth and development of Cotylurus sp. (Trematoda) in the domestic chick and on the chorioallantois. In: Program and Abstracts, 51st Annual Meeting, Am. Soc. Parasitol., p. 59 (1976)
Fried, B., Grigo, K.L.: Infectivity and excystation of the metacercaria of Echinoparyphium flexum (Trematoda). Proc. Penn. Acad. Sci. 49, 79–81 (1975)
Fried, B., Penner, L.R.: Studies on ocular trematodiasis. II. Growth of Philophthalmus sp. in vivo and on the chorioallantois of chicks. J. Parasitol. 47 (4, sect. 2), 31–32 (1961)
Fried, B., Roth, R.M.: In vitro excystment of the metacercaria of Parorchis acanthus. J. Parasitol. 60, 465 (1974)
Halton, D.W.: Studies on glycogen deposition in trematoda. Comp. Biochem. Physiol. 23, 113–120 (1967)
Howell, M.J.: Excystment and in vitro cultivation of Echinoparyphium serratum. Parasitology 58, 583–597 (1968)
Howell, M.J.: Excystment of the metacercariae of Echinoparyphium serratum (Trematoda: Echinostomatidae). J. Helminthol. 44, 35–56 (1970)
Insler, G.D., Roberts, L.S.: A possible crowding factor in Hymenolepis diminuta. In: Program and Abstracts, 50th Annual Meeting, Am. Soc. Parasitol., p. 95 (1975)
McDaniel, J.S.: Excystment of Cryptocotyle lingua metacercariae. Biol. Bull. 130, 369–377 (1966)
Mohandas, A.: Artyfechinostomum sufrartyfex Lane, 1915 a synonym of Echinostoma malayanum Leiper, 1911 (Trematoda: Echinostomatidae). Acta Parasitol. Pol. 19, 361–368 (1971a)
Mohandas, A.: Contributions to the cercarial fauna of Kerala. Ph.D. Thesis, University of Kerala (1971b)
Muraleedharan, K., Pande, B.P.: Notes on an experimental infection of laboratory-raised chicks maintained on normal and deficient feeds with Echinoparyphium flexum and a coccidium. Indian J. Anim. Health 6, 197–213 (1967)
Nollen, P.M.: Studies on growth and infection of Philophthalmus megalurus (Cort, 1914) (Trematoda) in chicks. J. Parasitol. 57, 261–266 (1971)
Oser, B.L.: Hawk's physiological chemistry. 14th. New York, Toronto, Sydney, London: The Blackiston Division, McGraw-Hill Book Company 1965
Rai, D.N., Pande, B.P.: On the metacercaria in Viviparus bengalensis (Lamarck) race mandiensis Kobelt and observations on an experimental infection with the Echinostoma form. Z. Parasitenkd. 28, 264–276 (1967)
Rankin, J.S.: An ecological study of parasites of some North Carolina salamanders. Ecol. Monogr. 7, 171–269 (1937)
Raymont, J.E.G., Austin, J., Linford, E.: Biochemical studies on marine zoo-plankton. I. The biochemical composition of Neomysis integer. J. Cons. perm. int. Explor. Mer. 28, 354–363 (1964)
Ross, J.G.: Experimental infections of cattle with Fasciola hepatica: A comparison of low and high infection rates. Nature 208, 907 (1965)
Seifter, S., Dayton, S., Novic, B., Muntwyler, E.: The estimation of glycogen with the anthrone reagent. Arch. Biochem. 25, 191–200 (1950)
Senger, C.M.: Notes on the growth, development and survival of two echinostome trematodes. Exp. Parasitol. 3, 491–496 (1954)
Smyth, J.D.: The physiology of trematodes. Edinburgh: Oliver and Boyd 1966
Sogandares-Bernal, F., Lumsden, R.D.: The heterophyid trematode Ascotyle leighi Burton, 1956 from the hearts of certain poeciliid and cyprinodont fishes. Z. Parasitenkd. 24, 3–12 (1964)
Ulmer, M.J.: Postharmostomum helicis (Leidy, 1847) Robinson, 1949 (Trematoda), its life history and a revision of the sub-family Brachylaeminae. Trans. Am. Microsc. Soc. 70, 189–238 (1951)
Watertor, J.L., Graham, D.W.: Effect of crowding on development and glycogen content of Telorchis corti Stunkard, 1915 (Trematoda: Telorchiidae). In: Program and Abstracts, 50th Annual Meeting, Am. Soc. Parasitol., p. 89 (1975)
Wikerhauser, T.: A rapid method for determining the viability of Fasciola hepatica metacercariae. Am. J. Vet. Res. 21, 895–897 (1960)
Willey, C.H.: The life history and bionomics of the trematode Zygocotyle lunata (Paramphistomidae). Zoologica 26, 65–88 (1941)
Ždárská, Z.: Methods for obtaining metacercariae from cysts in vitro. Folia Parasitol. 11, 343–345 (1964)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mohandas, A., Nadakal, A.M. In vivo development of Echinostoma malayanum Leiper, 1911 with notes on effects of population density, chemical composition and pathogenicity and in vitro excystment of the metacercaria (Trematoda: Echinostomatidae). Z. F. Parasitenkunde 55, 139–151 (1978). https://doi.org/10.1007/BF00384829
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00384829