Summary
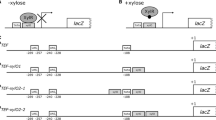
This study presents the first evidence that the 5′ promoter region of the Saccharomyces cerevisiae glyceraldehyde-3-phosphate dehydrogenase gene (G-3-PD) promoter will permit expression of an adjacent foreign gene. The S. cerevisiae G-3-PD promoter was linked to the herpes simplex virus — thymidine kinase (HSV-TK) gene in a shuttle plasmid capable of autonomous replication in both yeast and Escherichia coli. Since the HSV-TK gene promoter is not functional in yeast, yeast cells containing these plasmids will express the HSV-TK gene and synthesize thymidine kinase only if the yeast promoter fragment is fused to the HSV-TK gene in the proper orientation. The 5′ flanking sequences necessary for the expression of heterologous eukaryotic genes in S. cerevisiae are discussed.
Similar content being viewed by others
References
Alber T, Kawasaki G (1982) Nucleotide sequence of the triose phosphate isomerase gene of Saccharomyces cerevisiae. J Mol Appl Genet 1:419–434
Backman K (1980) A cautionary none on the use of certain restriction endonuclease with methylated substrates. Gene 11:169–171
Beggs JD, Van den Berg J, Van Ooyen A, Weissmann C (1980) Abnormal expression of chromosomal rabbit β-globin gene in Saccharomyces cerevisiae. Nature 283:835–840
Bennetzen JL, Hall BD (1982) The primary structure of the Saccharomyces cerevisiae gene for alcohol dehydrogenase I. J Biol Chem 257:3018–3025
Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucl Acids Res 7:1513–1523
Bisson LF, Thorner J (1977) Thymidine-5′-monophosphate requiring mutants of Saccharomyces cerevisiae are deficient in thymidylate synthetase. J Bacteriol 132:44–50
Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heyneker HL, Boyer HW, Corsa JH, Falkow S (1977) Construction and characterization of new cloning vehicles — II. A multipurpose cloning system. Gene 2:95–113
Bolivar F, Backman K (1979) Plasmids of Escherichia coli as cloning vectors. Methods in Enzymology 68:245–267
Boyer HW, Rouland-Dussolx D (1969) A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol 41:459–472
Brendel M, Fath WW (1974) Isolation and characterization of mutants of Saccharomyces cerevisiae auxotrophic and conditionally auxotrophic for 5′-dTMP. Z Naturforsch 29C:733–738
Brendel M, Fath WW, Laskowski W (1975) Isolation and characterization of mutants of Saccharomyces cerevisiae able to grow after inhibition of dTMP synthesis. Methods in Cell Biology 11:287–294
Britten RJ, Graham DE, Neufeld BR (1974) Analysis of repeating DNA sequences of reassociation. Methods in Enzymology 29:363–418
Cameron JR, Philippsen P, Davis RW (1977) Analysis of chromosomal integration and deletions of yeast plasmids. Nucl Acids Res 4:1429–1448
Clewell DB, Helinski DR (1972) Effect of growth conditions on the formation of the relaxation complex of supercoiled Col El deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol 110:1135–1146
Cohen JD, Eccleshall TR, Needleman RB, Federoff H, Buchferer BA, Marmur J (1980) Functional expression in yeast of the Escherichia coli plasmid gene coding for chloramphenicol acetyltransferase. Proc Natl Acad Sci USA 77:1078–1082
Colbere-Garapin F, Chousterman S, Horodniceanu F, Kourilsky P, Garapin AC (1979) Cloning of the active thymidine kinase gene of herpes simplex virus type 1 in Escherichia coli K-12. Proc Natl Acad Sci USA 76:3755–3759
Davis RW, Thomas M, Cameron J, St John TP, Scherer S, Padgett RA (1980) Rapid DNA isolations for enzymatic and hybridization analysis. Methods in Enzymology 65:404–411
Dobson MJ, Tuite MF, Roberts NA, Kingsman AJ, Kingsman SM (1982) Conservation of high efficiency promoter sequences in Saccharomyces cerevisiae. Nucl Acids Res 10:2625–2637
El Kouni MH, Sungman C (1981) A simple radioisotopic assay for nucleoside kinases employing alumina for separation of nucleoside and nucleotides. Anal Biochem 111:67–71
Gallwitz D, Perrin F, Seidel R (1981) The actin gene in yeast Saccharomyces cerevisiae: 5′ and 3′ end mapping, flanking and putative regulatory sequences. Nucl Acids Res 9:6339–6350
Grivell AR, Jackson JF (1968) Thymidine kinase: Evidence for its absence from neurospora crassa and some other micro-organisms and the relevance of this to the specific labeling of deoxyribonucleic acid. J Gen Microbiol 54:307–317
Grunstein M, Hogness D (1975) Colony hybridization: A method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci USA 72:3961–3965
Guo L-H, Wu R (1982) New rapid methods for DNA sequencing based on exonuclease III digestion followed by repair synthesis. Nucl Acids Res 10:2065–2084
Hartley JL, Donelson JE (1980) Nucleotide sequence of the yeast plasmid. Nature 286:860–865
Hattman S, Brooks JE, Masurerka M (1978) Sequence specificity of the P1 modification methylase (M Eco P1) and the DNA methylase (M Eco dam) controlled by the Escherichia coli dam gene. J Mol Biol 126:367–380
Henikoff S, Tatchell K, Hall BH, Nasmyth KA (1981) Isolation of a gene from Drosophila by complementation in yeast. Nature 289:33–37
Hinnen A, Hicks JB, Fink GR (1978) Transformation of yeast. Proc Natl Acad Sci USA 3:279–292
Hitzeman RA, Hagie FE, Levine HL, Goeddel DV, Ammerer G, Hall BD (1981) Expression of a human gene for interferon in yeast. 293:717–722
Hitzeman RA, Hagie FE, Hayflick JS, Chen CY, Seeburg PH, Derynck R (1982) The primary structure of Saccharomyces cerevisiae gene for 3-phosphoglycerate kinase. Nucl Acids Res 10:7791–7808
Hooland MJ, Holland JP (1979a) Isolation and characterization of a gene coding for glyceraldehyde-3-phosphate dehydrogenase from Saccharomyces cerevisiae. J Biol Chem 254:5466–5474
Holland JP, Holland MJ (1979b) The primary structure of a glyceraldehyde-3-phosphate dehydrogenase gene from Saccharomyces cerevisiae. J Biol Chem 254:9839–9845
Holland JP, Holland MJ (1980) Structural comparison of two nontandemly repeated yeast glyceraldehyde-3-phosphate dehydrogenase genes. J Biol Chem 255:2596–2605
Holland MJ, Holland JP, Thill GP, Jackson KA (1981) The primary structures of two yeast enolase genes. Homology between the 5′ noncoding flanking regions of yeast enolase and glyceraldehyde-3-phosphate dehydrogenase genes. J Biol Chem 256:1385–1395
Jansen S, Wittie I, Megnet R (1973) Mutants for the specific labeling of DNA in Saccharomyces cerevisiae. Biochem Biophys Acta 299:681–685
Johnson PH, Grossman LI (1977) Electrophoresis of DNA in agarose gel optimizing separations of conformational isomers of double- and single-stranded DNA. Biochemistry 16:4217–4225
Kiss GB, Cornish KV, Pearlman RE, Friesen JD (1980) The herpes simplex virus thymidine kinase gene is not expressed in Saccharomyces cerevisiae. Recombinant DNA Technical Bulletin 3:21–24
Kowalski D (1980) Fluorescence spot tests for DNA endonuclease ligase and topoisomerase activities. Anal Biochem 107:311–313
Kozak M (1981) Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucl Acids Res 9:5233–5252
Lehle L, Tanner W (1978) Biosynthesis and characterization of large dolichyl diphosphate-linked oligosaccharides in Saccharomyces cerevisiae. Biochem Biophys Acta 539:218–229
Little JG, Haynes RH (1979) Isolation and characterization of yeast mutants auxotrophic for 2′-deoxythymidine-5′-monophosphate. Mol Gen Genet 168:141–151
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory, New York
Marko MA, Chippafield R, Birnboim HC (1982) A procedure for the large-scale isolation of highly purified plasmid DNA using alkaline extraction and binding to glass powder. Anal Biochem 121:382–387
McNeill JB, Friesen JD (1981) Expression of the herpes simplex virus thymidine kinase gene in Saccharomyces cerevisiae. Mol Gen Genet 184:386–393
McKnight SL, Crose C, Kingsbury R (1979) Introduction of isolated DNA sequences into cultured eukaryotic cells. Carnegie Inst Washington Yearbook 78:56–61
McKnight SL (1980) The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucl Acids Res 8:5949–5964
McKnight SL, Gavis ER, Kingsbury R, Axel R (1981) Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: Identification of an upstream control region. Cell 25:385–398
McKnight SL, Kingsbury R (1982) Transcriptional control signals of a eukaryotic protein-coding gene. Science 217:316–324
Mercereau-Puijalon O, Lacroute F, Kourilsky P (1980) Synthesis of a chicken ovalbumin-like protein in the yeast Saccharomyces cerevisiae. Gene 11:163–167
Miller J (1974) Experiments in molecular genetics. Cold Spring Harbor Laboratory, New York
Norgard MW, Keem K, Monohan JJ (1978) Factors affecting the transformation of Escherichia coli strain chi 1776 by pBR322 plasmid DNA (Recombinant DNA; molecular cloning; cycloserine, vector DNA uptake). Gene 3:279–292
Ratzkin B, Carbon J (1977) Functional expression of cloned yeast DNA in Escherichia coli. Proc Natl Acad Sci USA 74:487–491
Sherman F, Fink GR, Hicks JB (1979) Methods in yeast genetics. Cold Spring Harbor Laboratory, p 61–62: 92 for regeneration agar
Smiley JR, Swan H, Pater MM, Pater A, Halpern ME (1983) Positive control of the herpes simplex virus thymidine kinase gene requires upstream DNA sequences. J Virol 47:301–310
Smith M, Leung DW, Gillam S, Astell CR (1979) Sequence of the gene for the iso-1-cytochrome c in Saccharomyces cerevisiae. Cell 16:753–761
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Struhl K, Stinchcomb DT, Scherer S, David RW (1979) High frequency transformation of yeast: Autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci USA 76:1035–1039
Taylor JM, Illmensee R, Summers J (1976) Efficient transcription of RNA into DNA by avian sarcoma virus polymerase. Biochem Biophys Acta 442:324–330
Ullrich A, Shine J, Chirgwin J, Pictet R, Tischer E, Rutter WJ, Goodman HM (1977) Rat insulin genes: Construction of plasmids containing the coding sequences. Science 196:1313–1319
Waechter CJ (1976) The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem 45:95–112
Wickner RB (1974) Mutants of Saccharomyces cerevisiae that incorporate deoxythymidine-5′-phosphate into deoxyribonucleic acid in vivo. J Bacteriol 117:252–260
Wieslander L (1979) A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem 98:305–309
Wigler M, Silverstein S, Lee LS, Pellicer A, Cheng YC, Axel R (1977) Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell 11:223–232
Author information
Authors and Affiliations
Additional information
Communicated by G. Fink
Rights and permissions
About this article
Cite this article
Zhu, XL., Ward, C. & Weissbach, A. Control of Herpes simplex virus thymidine kinase gene expression in Saccharomyces cerevisiae by a yeast promoter sequence. Molec. Gen. Genet. 194, 31–41 (1984). https://doi.org/10.1007/BF00383493
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00383493