Abstract
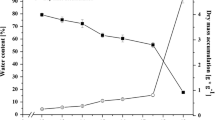
It was to be shown whether during the biogenesis of microbodies some of their components were already present in the cell prior to the organelle's assembly. To this end, the occurrence and properties of catalase in soluble and particular fractions of ripening cucumber seeds were examined. Homogenates of seeds from ripening fruits were fractionated by isopycnic density gradient centrifugation, and thus catalase was found in three different fractions: as a soluble enzyme in the gradient supernatant, as a membrane fraction at density d=1.18 kg l-1, and in association with microbodies. In the early steps of seed formation, catalase was detected at density d=1.18 kg l-1 and in the gradient supernatant. At a later stage of seed maturation, however, catalase was primarily associated with microbodies which exhibited an equilibrium density of d=1.23 kg l-1. M r as well as subunit M r of catalase were determined, and their close immunological relationship to leaf peroxisomal catalase and glyoxysomal catalase was demonstrated. Biosynthesis of catalase at different stages of seed maturation was investigated by in vivo labeling with l-[35S]methionine, l-[14C]leucine and δ-[3H]aminolaevulinic acid. Electrophoretic analysis of de novo synthesized catalase subunits revealed the occurrence of a heavy form (M r 57,500) in the soluble fraction; this form was preferentially labeled. A light form, M r 53,500, was detected in microbodies and also in the soluble fraction. The findings lend support to the hypothesis that the rate of catalase synthesis is highest in an early stage of seed formation, when globulins have already been formed, but before de novo synthesis of malate synthase has commenced. Prior to microbody assembling, a cytoplasmic pool of catalase was labeled.
Similar content being viewed by others
Abbreviations
- EDTA:
-
Na2-ethylenediaminotetraacetate
- Hepes:
-
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid
- M r :
-
molecular weight
References
Ammerer, G., Ruis, H.: Cell-free synthesis of Saccharomyces cerevisiae catalase T. FEBS Lett. 99, 242–246 (1979)
Betsche, T., Gerhardt, B., Apparent catalase synthesis in sunflower cotyledons during the change in microbody function. Plant Physiol. 62, 590–597 (1978)
Busch, M.: Stereochemische Studien an Hydrazonen von Dithiokohlensäureestern. I. J. Prakt. Chem. 93, 25–72 (1916)
Choinski, J.S., Trelease, R.N.: Control of enzyme activities in cotton cotyledons during maturation and germination. Plant Physiol. 62, 141–145 (1978)
Clark, R.W.: Calculation of s20, w values using ultracentrifuge sedimentation data from linear sucrose gradients, an imporved, simplified method. Biochim. Biophys. Acta 428, 269–274 (1976)
Drumm, H., Schopfer, P.: Effect of phytochrome on development of catalase activity and isoenzyme pattern in mustard (Sinapis alba L.) seedlings. A reinvestigation. Planta 120, 13–30 (1974)
Gerhardt, B., Betsche, T.: The change of microbodies from glyoxysomal to peroxisomal function within fatty greening cotyledons: hypotheses, results problems. Ber. Dtsch. Bot. Ges. 89, 321–324 (1976)
Gerhardt, B.: Microbodies/Peroxisomen pflanzlicher Zellen. Wien, New York: Springer 1978
Goldmann, B.M., Blobel, G.: Biogenesis of peroxisomes: Intracellular site of synthesis of catalase and uricase. Proc. Natl. Acad. Sci. USA 75, 5066–5070 (1978)
Gregory, E.M., Fridovich, I.: Visualization of catalase on acrylamide gels. Anal. Biochem. 58, 57–62 (1974)
Köller, W., Kindl, H.: Glyoxylate cycle enzymes of the glyoxysomal membrane from cucumber cotyledons. Arch. Biochem. Biophys. 181, 236–248 (1977)
Köller, W., Kindl, H.: The appearance of several malate synthase containing cell structures during the stage of glyoxysome biosynthesis. FEBS Lett. 88, 83–86 (1978)
Köller, W., Frevert, J., Kindl, H.: Albumins, glyoxysomal enzymes and globulins in dry seeds of Cucumis sativus: qualitative and quantitative analysis. Hoppe-Seyler's Z. Physiol. Chem. 360, 167–176 (1979a)
Köller, W., Frevert, J., Kindl, H.: Incomplete glyoxysomes appearing at a late stage of maturation of cucumber seeds. Z. Naturforschung 34c, 1232–1236 (1979b)
Lamb, J.E., Riezman, H., Becker, W.M.: Regulation of glyoxysomal enzymes during germination of cucumber. 2. Isolation and immunological detection of isocitrate lyase and catalase. Plant Physiol. 62, 754–760 (1978)
Lazarow, P.B., de Duve, C.: The synthesis and turnover of rat liver peroxisomes. IV. Biochemical pathway of catalase synthesis. J. Cell Biol. 59, 491–506 (1973a)
Lazarow, P.B., de Duve, C.: The synthesis and turnover of rat liver peroxisomes. V. Intracellular pathway of catalase synthesis. J. Cell Biol. 59, 507–524 (1973b)
Ludwig, B., Kindl, H.: Plant microbody proteins, II. Purification and characterization of the major protein component (SP-63) of peroxisome membranes. Hope-Seyler's Z. Physiol. Chem. 357, 177–186 (1976)
Okabe, T., Taniguchi, E., Maekawa, K.: Syntheses of 5-Triazolo[4,3-a]pyrimidine derivatives Agric. Biol. Chem. 37, 441–443 (1973)
Parikh, I., March, S., Cuatrecasas, P.: Topics in the methodology of substitution reactions with agarose. In: Methods in Enzymology, vol. 34, pp. 72–102, Jakoby, W.B., Wilchek, M., eds. New York: Academic Press 1974
Quail, P.H., Scandalios, J.G.: Turnover of genetically defined catalase isozymes in maize. Proc. Natl. Acad. Sci. USA 68, 1402–1406 (1974)
Robbi, M., Lazarow, P.B.: Synthesis of catalase in two cell-free protein-synthesizing systems and in rat liver. Proc. Natl. Acad. Sci. USA 75, 4344–4348 (1978)
Schiefer, S., Teifel, W., Kindl, H.: Plant microbody proteins I. Purification and characterization of catalase from leaves of Lens culinaris. Hoppe-Seyler's Z. Physiol. Chem. 357, 163–175 (1976)
Zimniak, P., Hartter, E., Woloszczyk, W., Ruis, H.: Catalase biosynthesis in yeast: Formation of catalase A and catalase T during oxygen adaptation of Saccharomyces cerevisiae. Eur. J. Biochem. 71, 393–398 (1976)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kindl, H., Schiefer, S. & Löffler, H.G. Occurrence and biosynthesis of catalase at different stages of seed maturation. Planta 148, 199–207 (1980). https://doi.org/10.1007/BF00380027
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00380027