Summary
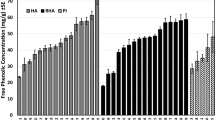
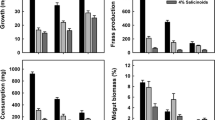
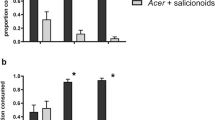
Papilio glaucus subspecies, hybrids and backcrosses exhibit greatly different abilities to use quaking aspen (Populus tremuloides) and other members of the Salicaceae as host plants. This study was conducted to test the hypotheses that phenolic glycosides account for the differences in larval performance, and that differential performance is correlated with differential larval esterase activities. To test the hypotheses we conducted first instar survival trials and fourth (penultimate) instar feeding trials with tremulacin, a phenolic glycoside. We also conducted assays of β-glucosidase, esterase, and glutathione transferase activities, using midgut enzyme preparations from fifth instars. First instar survival on the tremulacin treated diet generally improved with a higher proportion of Papilio glaucus canadensis genes in the genotype, although survival in one backcross treatment was surprisingly low. Penultimate instars of P.g. glaucus and P.g. australis fed tremulacin treated black cherry leaves experienced a severe reduction in growth rate relative to larvae fed control leaves. This seriously suppressed growth was partially due to reduced consumption rates and reduced conversion efficiencies, however, approximate digestibility was not affected. In contrast, P. g. canadensis and hybrids showed no differences in growth rates between tremulacin treated and control leaves. Reciprocal backcrosses of hybrids with P. g. glaucus resulted in slightly suppressed growth on treated versus control leaves. The results suggest that after a certain threshold, increased proportions of P. g. glaucus genes resulted in poorer growth performance with tremulacin in the diet. Soluble esterase activities generally increased with the proportion of Papilio glaucus canadensis genes in the genotype, and paralleled overall trends in larval survival and feeding performance. We conclude that phenolic glycosides such as tremulacin are responsible for differential performance of Papilio glaucus subspecies, hybrids and backcrosses fed plants in the Salicaceae, and that detoxification of phenolic glycosides by midgut esterase explains why some Papilio glaucus genotypes can effectively utilize these plants.
Similar content being viewed by others
References
Berenbaum M (1983) Coumarins and caterpillars: a case for coevolution. Evolution 37:163–179
Brower LP (1958) Larval foodplant specificity in butterflies of the Papilio glaucus group. Lepidoptera News 12:103–114
Brown KS, Damman AJ, Feeny PP (1980) Troidine swallowtails (Lepidoptera: Papilionidae) in southeastern Brazil: natural history and foodplant relationships. J Res Lepid 19:199–226
Curtis JT (1959) The vegetation of Wisconsin. University of Wisconsin Press, Madison Wisconsin
Dethier VG (1954) Evolution of feeding preferences in phytophagous insects. Evolution 8:33–54
Devonshire AL (1977) The properties of a carboxylase from the peach-potato aphid, Myzus persicae (Sulz), and its role in conferring insecticide resistance. Biochem J 167:675–683
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution 18:586–608
Feeny P, Rosenberry L, Carter M (1983) Chemical aspects of oviposition behavior in butterflies. In: Ahmad S (ed) Herbivorous insects: host-seeking behavior and mechanisms. Academic Press, New York NY, pp 27–76
Grabstein EM, Scriber JM (1982a) The relationship between restriction of host plant consumption and post-ingestive utilization of biomass and nitrogen in Hyalophora cecropia. Entomol Exp Appl 31:202–210
Grabstein EM, Scriber JM (1982b) Host plant utilization as affected by prior feeding experience. Entomol Exp Appl 32:262–268
Hagen RH (1986) The evolution of host-plant use by the tiger swallowtail butterfly, Papilio glaucus. PhD Dissertation. Cornell University, Ithaca, NY
Hancock DL (1983) Classification of the Papilionidae (Lepidoptera): a phylogenetic approach. Smithersia 2:1–48
Lederhouse RC, Ayres MP, Scriber JM (1989) Artificial diet affects male virility in Papilio glaucus. Functional Ecol (in press)
Lindroth RL (1988) Hydrolysis of phenolic glycosides by midgut β-glucosidases in Papilio glaucus subspecies. Insect Biochem 18:789–792
Lindroth RL (1989a) Biochemical detoxication: mechanism of differential tiger swallowtail tolerance to phenolic glycosides. Oecologia (in press)
Lindroth RL (1989b) Host plant alteration of detoxication activity in Papilio glaucus glaucus. Entomol Exp Appl 50:29–35
Lindroth RL, Peterson SS (1988) Effects of plant phenols on performance of southern armyworm larvae. Oecologia 75:185–189
Lindroth RL, Scriber JM, Hsia MTS (1986) Differential responses of tiger swallowtail subspecies to secondary metabolites from tulip tree and quaking aspen. Oecologia 70:13–19
Lindroth RL, Hsia MTS, Scriber JM (1987) Characterization of phenolic glycosides from quaking aspen (Populus tremuloides). Biochem Syst Ecol 15:677–680
Lindroth RL, Scriber JM, Hsia MTS (1988) Chemical ecology of the tiger swallowtail: mediation of host use by phenolic glycosides. Ecology 69:814–822
Miller JS (1987) Host-plant relationships in the Papilionidae (Lepidoptera): parallel cladogenesis or colonization? Cladistics 3:105–120
Munroe E (1960) The generic classification of the Papilionidae. Can Entomol [Suppl] 17:1–51
Palo RT (1984) Distribution of birch (Betula spp.), willow (Salix spp.), and poplar (Populus spp.) secondary metabolites and their potential role as chemical defense against herbivores. J Chem Ecol 10:499–520
Schacterle GR, Pollack RL (1973) A simplified method for quantitative assay of small amounts of protein in biologic material. Anal Biochem 51:654–655
Scriber JM (1973) Latitudinal gradients in larval feeding specialization of the World Papilionidae. Psyche 80:355–373
Scriber JM (1977) Limiting effects of low leaf-water content on the nitrogen utilization, energy budget, and larval growth of Hyalophora cecropia (Lepidoptera Saturniidae). Oecologia 28:269–287
Scriber JM (1981) Sequential diets, metabolic costs and growth of Spodoptera eridania feeding upon dill, lima bean, and cabbage. Oecologia 51:175–180
Scriber JM (1982a) Foodplants and speciation in the Papilio glaucus group. In: Visser JH, Minks AK (eds) Proc 5th International Symp on Insect Plant Relationships. PUDOC, Wageningen, Netherlands, pp 307–314
Scriber JM (1982b) The behavior and nutritional physiology of southern armyworm larvae as a function of plant species consumed in earlier instars. Entomol Exp Appl 31:359–369
Scriber JM (1984) Larval foodplant utilization by the World Papilionidae (Lepidoptera): latitudinal gradients reappraised. Tokurana (Acta Rhopalocerologica) 2:1–50
Scriber JM (1986) Allelochemicals and alimentary ecology: heterosis in a hybrid zone? In: Brattsten L, Ahmad S (eds) Molecular mechanisms in insect plant associations. Plenum Press, New York, NY, pp 43–71
Scriber JM (1988) Tale of the tiger: beringial biogeography, bionomial classification, and breakfast choices in the Papilio glaucus complex of butterflies. In: Spencer KC (ed) Chemical mediation of coevolution. Academic Press, New York NY, pp 240–301
Scriber JM, Ayres M (1988) Leaf chemistry as a defense against insects. Institute of Scientific Information. Atlas of Science. Plants and Animals 1:117–123
Scriber JM, Hagen R, Lederhouse RC (1989) Foodplants and evolution within the Papilio glaucus and Papilio troilus species groups (Lepidoptera: Papilionidae). In: Price PW, Lewinsohn TM, Benson WW, Fernandes GW (eds) Evolutionary ecology of plant-animal interactions: tropical and temperate perspectives. John Wiley, New York (in press)
Waldbauer GP (1968) The consumption and utilization of food by insects. Adv Insect Physiol 5:229–288
Wilkinson L (1987) SYSTAT: The System for Statistics. SYSTAT Inc Evanston IL
Winer BJ (1962) Statistical principles in experimental design. McGraw-Hill, New York, p 672
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Scriber, J.M., Lindroth, R.L. & Nitao, J. Differential toxicity of a phenolic glycoside from quaking aspen to Papilio glaucus butterfly subspecies, hybrids and backcrosses. Oecologia 81, 186–191 (1989). https://doi.org/10.1007/BF00379804
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00379804