Summary
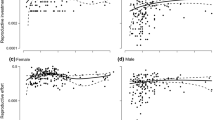
Populations of Lomatium grayi (Umbelliferae), an andromonoecious, perennial herb, differe in growth rates, flowering frequency, and survivorship. Effects of these different life histories on the ontogeny of sex expression were analyzed for plants from two populations grown from seedlings in a common garden and monitored for six years. Plants from Smoot Hill, Washington grew faster, had a higher probability of flowering at each age and size after the first year of growth, and a higher probability of flowering repeatedly among years than did plants from Clarkston, Washington. The proportion of plants producing some hermaphroditic flowers increased with plant size in both populations. Smoot Hill plants, however, were more likely to begin flowering as small, staminate plants than Clarkston plants. Clarkston plants did not begin flowering until they were older and larger, and most of these plants produced some hermaphroditic flowers when they began reproduction. The positive association between production of hermaphroditic flowers and both plant size and age was consistent with the hypothesis that hermaphroditic flowers are more costly to produce than staminate flowers.
Although the populations did not differ in the total number of flowers per plant produced at any age or size, Smoot Hill plants consistently produced a lower percentage of hermaphroditic flowers than Clarkston plants at larger sizes and later ages. Consequently, selection for faster growth rates and higher flowering frequency at small sizes and early ages may have favored the more staminate-biased sex ratios in the Smoot Hill population.
Similar content being viewed by others
References
Antonovics J, Primack RB (1982) Experimental ecological genetics in Plantago. VI. The demography of seedling transplants of P. lanceolata. J Ecol 70:55–75
Bawa KS, Beach JH (1981) Evolution of sexual systems in plants. Ann Missouri Bot Gard 68:254–274
Bazzaz FA, Harper JL (1976) Relationship between plant weight and number in mixed populations of Sinapsis arvensis (L.) Raben L. and Lepidium sativum L. J Appl Ecol 13:211–216
Bell CR (1971) Breeding systems and floral biology of the Umbelliferae or evidence for specialization in unspecialized flowers. In: Heywood VH (ed) The biology and chemistry of the Umbelliferae. Academic Press, New York, pp 93–107
Bertin RI (1982a) The ecology of sex expression in red buckeye. Ecology 63:445–456
Bertin RI (1982b) The evolution and maintenance of andromonoecy. Evol Theory 6:25–32
Bierzychudek P (1984) Determinants of gender in Jack-in-the-pulpit: the influence of plant size and reproductive history. Oecologia (Berlin) 65:14–18
Braak JP, Kho YO (1958) Some observations on the floral biology of the carrot (Daucus carota L.). Euphytica 7:131–139
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton, New Jersey
Charnov EL, Bull J (1977) When is sex environmentally determined? Nature 266:828–830
Cruden RW (1976) Intraspecific variation in pollen-ovule ratios and nectar secretion-preliminary evidence of ecotypic adaptation. Ann Missouri Bot Gard 63:277–289
Daubenmire R (1970) Steppe vegetation of Washington, Washington Agric Exp Stn Tech Bull 62
Freeman DC, McArthur ED, Harper KT, Blauer AC (1981) Influence of environment on the floral sex ratio of monoecious plants. Evolution 35:194–197
Ghiselin MT (1974) The economy of nature and the evolution of sex. University of California Press, Berkeley
Hardin E (1929) The flowering and fruiting habits of Lomatium. Research Studies, State College of Washington 1:15–25
Harper JL (1977) Population biology of plants. Academic Press, New York
Hendrix SD (1984) Reactions of Heracleum lanatum to floral herbivory by Depresaria pastinacella. Ecology 65:191–197
Hendrix SD, Trapp EJ (1981) Plant-herbivore interactions: insect induced changes in host plant sex expression and fecundity. Oecologia (Berlin) 49:119–122
Lindsey AH (1984) Reproductive biology of Apiaceae. I. Floral visitors to Thaspium and Zizia and their importance in pollination. Am J Bot 71:375–387
Lloyd DG (1979) Parental strategies of angiosperms. New Zealand J Bot 17:595–606
Lloyd DG (1980) The distributions of gender in four angiosperms illustrating two evolutionary pathways to dioecy. Evolution 33:123–134
Lloyd DG, Bawa KS (1984) Modification of the gender of seed plants in varying conditions. Evol Biol 17:255–338
Loveless MD, Hamrick JL (1984) Ecological determinants of genetic structure in plant populations. Ann Rev Ecol Syst 15:65–95
Lovett Doust J (1980a) Floral sex ratios in andromonoecious Umbelliferae. New Phytol 85:265–273
Lovett Doust J, Cavers PB (1982) Sex and gender dynamics in Jack-in-the-pulpit, Arisaema triphyllum (Araceae). Ecology 63:797–808
Lovett Doust J, Harper JL (1980) The resource costs of gender and maternal support in an andromonoecious umbellifer, Smyrnium olusatrum L. New Phytol 85:251–264
McArthur ED (1977) Environmentally induced changes of sex expression in Atriplex canescens. Heredity 38:97–103
Policansky D (1981) Sex choice and the size advantage model in Jack-in-the-pulpit (Arisaema triphyllum). Proc Natl Acad Sci, USA 78:1306–1308
Ponomarev AN (1960) Concerning protandry in Umbelliferae. Doklady Akademii Nauk. SSSR 135:750–752
Primack RB, Lloyd DG (1980) Andromonoecy in the New Zealand montane shrub manuka, Leptospermum scoparium (Myrtaceae). Am J Bot 67:361–368
SAS Institute Inc (1985) SAS user's guide: statistics. Version 5th ed. SAS Institute, Cary, NC
Schemske DW (1984) Population structure and local selection in Impatiens pallida (Balsaminaceae), a selfing annual. Evolution 38:817–832
Schlessman MA (1982) Expression of andromonoecy and pollination of tuberous lomatiums (Umbelliferae). Syst Bot 7:134–149
Singh VP, Ramanujam S (1973) Expression of andromonoecy in coriander, Coriandrum sativum L. Euphytica 22:181–188
Solomon BP (1986) Sexual allocation and andromonoecy: resource investment in male and hermaphroditic flowers of Solanum carolinense (Solanaceae). Am J Bot 73:1215–1221
Thompson JN (1982) Interaction and coevolution. Wiley-Interscience, New York
Thompson JN (1983a) Selection of plant parts by Depressria multifidae (Lep., Oecophoridae) on its seasonally-restricted hostplant, Lomatium grayi (Umbelliferae). Ecol Entomol 8:203–211
Thompson JN (1983b) Partitioning of variance in demography: within-patch differences in herbivory, survivial, and flowering of Lomatium farinosum (Umbelliferae). Oikos 40:315–317
Thompson JN (1984) Variation among individual seed masses in Lomatium grayi (Umbelliferae) under controlled conditions: magnitude and partitioning of the variance. Ecology 65:626–631
Thompson JN (1985a) Within-patch dynamics of life histories, populations, and interactions: selection over time in small spaces. In: Pickett STA, White PS (eds) The ecology of natural disturbance and patch dynamics. Academic Press, New York, pp 253–264
Thompson JN (1985b) Postdispersal seed predation on Lomatium spp. (Umbelliferae): variation among individuals and species. Ecology 66:1608–1616
Thompson JN, Moody ME (1985) Assessing probability of interaction in size-structured populations: Depressaria attack on Lomatium. Ecology 66:1597–1607
Venable DL (1984) Using intraspecific variation to study the ecological significance and evolution of plant life-histories. In: Dirzo R, Sarukhan J (eds) Perspectives on plant population ecology. Sinauer, Sunderland, MS, pp 166–187
Warner RR (1975) The adaptive significance of sequential hermaphroditism in animals. Am Nat 109:61–82
Warner RR, Hoffman SG (1980) Local population size as a determinant of mating system and sexual composition in two tropical marine fishes (Thalassoma spp.). Evolution 34:508–518
Watkinson AR, White JL (1986) Some life-history consequences of modular construction in plants. Phil Trans Roy Soc Lond B 313:31–52
Webb CJ (1979) Breeding systems and the evolution of dioecy in New Zealand Apioid Umbelliferae. Evolution 33:662–672
Williams CN, Thomas RL (1970) Observations on sex differentiation in the oil palm, Elias quineensis L. Ann Bot 34:957–963
Willson MF (1979) Sexual selection in plants. Amer Natur 113:777–790
Willson MF (1983) Plant reproductive ecology. Wiley, New York, NY
Willson MF, Burley N (1983) Mate choice in plants. Princeton University Press, Princeton, NJ
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thompson, J.N. The ontogeny of flowering and sex expression in divergent populations of Lomatium grayi . Oecologia 72, 605–611 (1987). https://doi.org/10.1007/BF00378989
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00378989