Summary
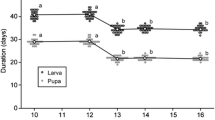
Chromosomally polymorphic populations of Drosophila mojavensis from Baja California feed and breed on agria cactus, Stenocereus gummosus; whereas, monomorphic Arizona populations are associated exclusively with organ pipe cactus, S. thurberi. The effects of this host plant shift in expanding the kinds of feeding and breeding sites were assessed by manipulating larval density and recording differences in egg to adult development time and viability, and adult thorax size in both populations on artificially rotted substrates of both cactus species. Older agria rots increased development time but had no effect on viability. Organ pipe rots were qualitatively poorer substrates than agria rots for both monomorphic and polymorphic populations of D. mojavensis, especially at higher larval densities causing longer egg to adult development times, lower viabilities, and smaller thorax sizes than agria.
The Baja population expressed shorter development times, higher viabilities, and smaller thorax sizes than the Arizona population on both cactus substrates. No evidence for cactus host race formation was found. The Baja population was less sensitive to increasing larval densities for all fitness characters studied on both cactus substrates indicating greater developmental homeostasis than in the monomorphic Arizona population. These data support the hypothesized central-marginal population structure within this species coincident with the distribution of host plants and lend insight into the process of adaptive divergence at different life history stages caused by host plant shifts.
Similar content being viewed by others
References
Atkinson WD (1979) A comparison of the reproductive strategies of domestic species of Drosophila. J Anim Ecol 48:53–64
Brussard PF (1984) Geographical patterns and environmental gradients: The central-marginal model in Drosophila revisited. Ann Rev Ecol Syst 15:25–64
Carson HL (1959) Conditions which promote or retard the formation of species. Cold Spr Harb Symp Quant Biol 24:87–105
Chiang HC, Hodson AC (1950) An analytical study of population growth in Drosophila melanogaster. Ecol Monog 20:173–206
Dobzhansky Th (1951) Genetics and the Origin of Species. 3rd ed., Columbia, New York
Fellows DP, Heed WB (1972) Factors affecting host plant selection in desert-adapted Drosophila. Ecology 53:850–858
Fogleman JC, Starmer WT (1985) Analysis of community structure of yeasts associated with the decaying stems of cactus. III. Stenocereus thurberi. Microb Ecol 11:165–173
Fogleman JC, Starmer WT, Heed WB (1981) Larval selectivity for yeast species by Drosophila mojavensis in natural substrates. Proc Natl Acad Sci USA 78:4435–4439
Fogleman JC, Starmer WT, Heed WB (1982) Comparisons of yeast flora from natural substrates and larval guts of southwestern Drosophila. Oecologia (Berlin) 52:187–191
Fogleman JC, Duperret SM, Kircher HW (1986) The role of phytosterols in host plant utilization by cactophilic Drosophila. Lipids 21:92–96
Futuyma DJ, Mayer GC (1980) Non-allopatric speciation in animals. Syst Zool 29:254–271
Futuyma DJ, Peterson SC (1985) Genetic variation, in the use of resources by insects. Ann Rev Entomol 30:217–238
Heed WB (1977) A new cactus-feeding but soil-breeding species of Drosophila (Diptera: Drosophilidae). Proc Ent Soc Wash 79:649–654
Heed WB (1978) Ecology and genetics of Sonoran Desert Drosophila. In: Brussard PF (ed) Ecological Genetics: The Interface. Springer, New York pp 109–126
Heed WB (1981) Central and marginal populations revisited. Dros Inf Serv 56:60–61
Heed WB (1982) The origin of Drosophila in the Sonoran desert. In: Barker JSF, Starmer WT (eds) Ecological Genetics and Evolution: The Cactus-Yeast-Drosophila Model System. Academic Press, New York p 65–80
Heed WB, Mangan RL (1986) Community ecology of the Sonoran Desert Drosophila. In: Ashburner M, Carson HL, Thompson JN (eds) The Genetics and Biology of Drosophila. vol 3e. Academic Press, New York pp 311–345
Helwig JT, Council KA (1979) SAS User's Guide 1979 Edition. SAS Institute, Cary, North Carolina
Hiraizumi Y (1961) Negative correlation between rate of development and female fertility in Drosophila melanogaster. Genetics 46:615–624
Jaenike J (1981) Criteria for ascertaining the existence of host races. Am Nat 117:830–834
Jermy T (1984) Evolution of insect/host plant relationships. Am Nat 124:609–630
Johnson WR (1980) Chromosomal polymorphism in desert-adapted Drosophila mojavensis. Phd Thesis, University of Arizona, Tucson, Arizona
Kircher HW (1982) Chemical composition of cacti and its relationship to Sonoran Desert Drosophila. In: Barker JSF, Starmer WT (eds) Ecological Genetics and Evolution: The Cactus-Yeast-Drosophila Model System. Academic Press, New York pp 143–158
Lerner IM (1954) Genetic Homeostasis. Dover, New York
Lewontin RC (1957) The adaptations of populations to varying environments. Cold Spr Harb Symp Quant Biol 22:395–408
Lofdahl KL (1985) A quantitative genetic analysis of habitat selection behavior in the cactus-breeding species Drosophila mojavensis. PhD Thesis, University of Chicago, Chicago, Illinois
Mangan RL (1978) Competitive interactions and host plant specific Drosophila species. PhD Thesis, University of Arizona, Tucson, Arizona
Mangan RL (1982) Adaptations to competition in cactus breeding Drosophila. In: Barker JSF, Starmer WT (eds) Ecological Genetics and Evolution: The Cactus-Yeast-Drosophila Model System. Academic Press, New York pp 143–158
Marien D (1965) Selection for developmental rate in Drosophila pseudoobscura. Genetics 50:3–15
McFarquhar AM, Robertson FW (1963) The lack of evidence for coadaptation in crosses between geographical races of Drosophila subobscura (Coll.) Genet Res 4:104–131
Mettler LE (1963) D. mojavensis baja, a new form in the mulleri complex. Dros Inf Serv 28:57–58
Robertson FW (1957) Studies in quantitative inheritance. XI. Genetic and environmental correlation between body size and egg production in Drosophila melanogaster. J Genet 55:428–443
Sang JH (1956) The quantitative nutritional requirements of Drosophila melanogaster. J Exp Biol 33:45–72
Sang JH, Clayton GA (1957) Selection for larval development in Drosophila melanogaster. J Hered 48:265–270
Sokoloff A (1966) Morphological variation in natural populations of Drosophila pseudoobscura and Drosophila persimilis. Evolution 20:49–71
Spiess EB, Spiess LD (1966) Selection for rate of development and gene arrangement frequencies in Drosophila persimilis. II. Fitness properties at equilibrium. Genetics 53:695–708
Starmer WT (1982a) Analysis of community structure of yeasts associated with decaying stems of cactus. I. Stenocereus gummosus. Microb Ecol 8:71–81
Starmer WT (1982b) Associations and interactions among yeasts, Drosophila and their habitats. In: Barker JSF, Starmer WT (eds) Ecological Genetics and Evolution: The Cactus-Yeast-Drosophila Model System. Academic Press, New York pp 159–174
Tantaway AO, Mallah GS (1961) Studies on natural populations of Drosophila melanogaster. I. Heat resistance and geographical variation in Drosophila melanogaster and D. simulans. Evolution 15:132–144
Zouros E (1973) Genic differentiation, associated with the early stages of speciation in the mulleri sub-group of Drosophila. Evolution 27:601–621
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Etges, W.J., Heed, W.B. Sensitivity to larval density in populations of Drosophila mojavensis: Influences of host plant variation on components of fitness. Oecologia 71, 375–381 (1987). https://doi.org/10.1007/BF00378710
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00378710