Abstract
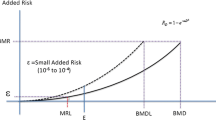
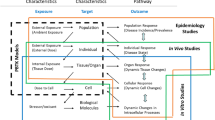
The National Research Council (NRC) recently published a report, Science and Judgment in Risk Assessment, that critiqued the current approaches to characterizing human cancer risks from exposure to chemicals. One issue raised in the report relates to the use of default options for quantitation of cancer risks. Default options are general guidelines that can be used for risk assessment when specific information about a chemical is absent. Research on 1,3-butadiene represents an interesting case study in which existing knowledge on this chemical indicates that two default options may no longer be tenable: (1) humans are as sensitive as the most sensitive animal species, and (2) the rate of metabolism is a function of body surface area rather than inherent species differences in metabolic capacity. Butadiene, a major commodity chemical used in the production of synthetic rubber, is listed as one of 189 hazardous air pollutants under the 1990 Clean Air Act Amendments. Butadiene is a carcinogen in rats and mice, with mice being substantially more sensitive than rats. The extent to which butadiene poses a cancer risk to humans exposed to this chemical is uncertain. Butadiene requires metabolic activation to DNA-reactive epoxides to exert its mutagenic and carcinogenic effects. Research is directed toward obtaining a better understanding of the cancer risks of butadiene in humans by evaluating species-dependent differences in the formation of the toxic butadiene epoxide metabolites, epoxybutene and diepoxybutane. The data include in-vitro studies on butadiene metabolism using tissues from humans, rats, and mice as well as experimental data and physiological model predictions for butadiene in blood and butadiene epoxides in blood, lung, and liver after exposure of rats and mice to inhaled butadiene. The findings suggest that humans are more like rats and less like mice regarding the formation of butadiene epoxides. The research approach employed can be a useful strategy for developing mechanistic and toxicokinetic data to supplant default options used in carcinogen risk assessments for butadiene.
Similar content being viewed by others
References
CW product focus: butadiene. Chemical Week, June 15, 1994:
U.S. Environmental Protection Agency (1992) National emission standards for hazardous air pollutants for source categories; organic hazardous air pollutants from the synthetic organic chemical manufacturing industry and seven other processes. Federal register 57 (252), Part II, 62608–62808
Fajen JM et al. (1993) Industrial exposure to 1,3-butadiene in monomer, polymer and end-user industries. In: Sora M, Peltonen K, Vainio H, Hemminski K (eds) Butadiene and styrene: assessment of health hazards. Lyon, International Agency for Research on Cancer, pp 3–13
Himmelstein MW et al. (1995) Toxicology and epidemiology of 1,3-butadiene. CRC critical reviews in toxicology (in press)
National Research Council (1994) Science and judgment in risk assessment. Washington, National Academy Press
Bond JA et al. (1995) Epidemiological and mechanistic data suggest that 1,3-butadiene will not be carcinogenic to humans at exposures likely to be encountered in the environment or workplace. Carcinogenesis 16:165–171
Huff JE et al. (1985) Multiple organ carcinogenicity of 1,3-butadiene in B6C3F1 mice after 60 weeks of inhalation exposure. Science 227:548–549
Melnick RL et al. (1990) Carcinogenicity of 1,3-butadiene in B6C3F1 mice at low exposure concentrations. Cancer Res 50:6592–6599
Owen PE et al. (1987) Inhalation toxicity studies with 1,3-butadiene. III. Two year toxicity/carcinogenicity study in rats. Am Ind Hyg Assoc J 48:407–413
Occupational Safety and Health Administration (1990) Occupational exposure to 1,3-butadiene: Proposed rule and notice of hearing. Federal Register 55 (155):32736–32826
Meester C de (1988) Genotoxic properties of 1,3-butadiene. Mutat Res 195:273–281
Meester C de et al. (1980) The mutagenicity of butadiene towards Salmonella typhimurium. Toxicol Lett 6:125–130
Tice RR et al. (1987) Comparative cytogenetic analysis of bone marrow damage induced in male B6C3F1 mice by multiple exposures to gaseous 1,3-butadiene. Environ Mutag 9:235–250
Cunningham MJ et al. (1986) In vivo sister chromatid exchange and micronucleus induction studies with 1,3-butadiene in B6C3F1 mice and Sprague-Dawley rats. Mutagenesis 1:449–452
Autio K et al. (1994) Induction of micronuclei in peripheral blood and bone marrow erythrocytes of rats and mice exposed to 1,3-butadiene by inhalation. Mutat Res 309:315–320
Conner MK et al. (1983) Induction and rapid repair of sister-chromatid exchanges in multiple murine tissues in vivo by diepoxybutane. Mutat Res 108:251–263
Sharief Y et al. (1986) Sister chromatid exchange and chromosome aberration analyses in mice after in vivo exposure to acrylonitrile, styrene, or butadiene monoxide. Environ Mutag 8:439–448
Duuren BL van et al. (1963) Carcinogenicity of epoxides, lactones, and peroxy compounds. J Nat Cancer Inst 31:41–55
Duuren BL van et al. (1966) Carcinogenicity of epoxides, lactones, and peroxy compounds. IV. Tumor response in epithelial and connective tissue in mice and rats. J Nat Cancer Inst 37:825–838
Cochrane JE, Skopek TR (1994) Mutagenicity of butadiene and its epoxide metabolites: 1. Mutagenic potential of 1,2-epoxybutene, 1,2,3,4-diepoxybutane and 3,4-epoxy-1,2-butanediol in cultured human lymphoblasts. Carcinogenesis 15:713–717
Sasiadek M et al. (1991a) Sister-chromatid exchanges induced by 1,3-butadiene and its epoxides in CHO cells. Mutat Res 263:47–50
Sasiadek M et al. (1991b) 1,3-Butadiene and its epoxides induce sister-chromatid exchanges in human lymphocytes in vitro. Mutat Res 261:117–121
Sorsa M et al. (1994) Human cytogenetic biomonitoring of occupational exposure to 1,3-butadiene. Mutat Res 309:321–326
Csanády GA et al. (1992) Comparison of the biotransformation of 1,3-butadiene and its metabolite, butadiene monoepoxide, by hepatic and pulmonary tissues from humans, rats and mice. Carcinogenesis, 13:1143–1153
Seaton MJ et al. (1995) Oxidation of 1,2-epoxy-3-butene to 1,2:3,4-diepoxybutane by cDNA expressed human cytochromes P450 2E1 and 3A4 and human, mouse, and rat liver microsomes. Carcinogenesis 16:2287–2293
Boogaard PJ et al. (1996) Glutathione conjugation of 1:2,3:4-diepoxybutane in human liver and rat and mouse liver and lung in vitro. Toxicol Appl Pharmacol 136:307–316
Himmelstein MW et al. (1994) Comparison of blood concentrations of 1,3-butadiene and butadiene epoxides in mice and rats exposed to 1,3-butadiene by inhalation. Carcinogenesis 15:1479–1486
Himmelstein MW et al. (1995) High concentrations of butadiene epoxides in livers and lungs of mice compared to rats exposed to 1,3-butadiene. Toxicol Applied Pharmacol 132:281–288
Thornton-Manning JR et al. (1996) Disposition of butadiene monoepoxide and butadiene diepoxide in various tissues of rats and mice following a low-level inhalation exposure to 1,3-butadiene. Carcinogenesis 16:1723–1731
Johanson G, Filser JG (1993) A physiologically based pharmacokinetic model for butadiene and its metabolite butadiene monoxide in rat and mouse and its significance for risk extrapolation. Arch Toxicol 93:151–163
Kohn MC, Melnick RL (1993) Species differences in the production and clearance of 1,3-butadiene metabolites: A mechanistic model indicates predominantly physiological, not biochemical control. Carcinogenesis 14: 619–628
Evelo CTA et al. (1993) Physiologically based toxicokinetic modeling of 1,3-butadiene lung metabolism in mice becomes more important at low doses. Environ Health Perspec 101:496–502
Medinsky MA et al. (1994) In vivo metabolism of butadiene by mice and rats: A comparison of physiological model predictions and experimental data. Carcinogenesis 15:1329–1340
Osterman-Golkar SM et al. (1993) Use of haemoglobin adducts for biomonitoring exposure to 1,3-butadiene. In: Sorsa M, Peltonen K, Vainio H, Hemminki K (eds) Butadiene and styrene: assessment of health hazards. Lyon, International Agency for Research on Cancer, pp 127–134
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bond, J.A., Himmelstein, M.W. & Medinsky, M.A. The use of toxicologic data in mechanistic risk assessment: 1,3-butadiene as a case study. Int Arch Occup Environ Health 68, 415–420 (1996). https://doi.org/10.1007/BF00377862
Issue Date:
DOI: https://doi.org/10.1007/BF00377862