Summary
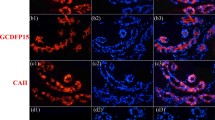
Human eccrine sweat gland ducts and benign and malignant eccrine poromas were studied for the expression of various cytokeratins (CK) and vimentin by applying immunoperoxidase and immunofluorescence microscopy to frozen or paraffin-embedded sections, and using two-dimensional gel electrophoresis and immunoblotting. In acrosyringia and dermal eccrine ducts, the luminal cells exhibited intense staining for CKs 1/10/11 and 19. The periluminal cell layers of acrosyringia contained CKs 1/10/11, while CK 5 was absent. In contrast, the basal cell layer of dermal ducts was only positive with the antibody against CK 5, i. e. a pattern resembling that seen in epidermal basal cells. CK 9 was detected only in keratinocytes peripherally surrounding acrosyringia. In benign poromas, gel electrophoresis revealed that CKs 5 and 14 were predominant, with CKs 6, 16 and 17 being minor components. At the immunohistochemical level CKs 1/10/11 and 19 could be further detected with varying frequency in scattered or clustered cells and/or duct-like structures. Occasionally, CK 9-positive cells were observed. Malignant poromas displayed a similar overall gel-electrophoretic pattern. Their immunohistochemical staining patterns were also similar to (albeit rather more variable than) those seen in benign poromas. Our results show that, with respect to their CK expression pattern, the majority of poroma cells resemble the basal cells of both the dermal ducts and the epidermis, while only minor and variable subpopulations acquire features present in ductal/acrosyringial luminal cells that would be indicative of poral differentiation. Thus, the matrix cells of poromas seem to be most closely related to basal cells located at the transition between the glandular epidermal ridge and dermal eccrine duct, being in no way analogous to the cells of the adult acrosyringium above the basal cell level.
Similar content being viewed by others
References
AchtstÄtter T, Hatzfeld M, Quinlan RA, Parmeles DC, Franke WW (1986) Separation of cytokeratin polypeptides by gel electrophoretic and Chromatographic techniques and their identification by immunoblotting. Methods Enzymol 134:355–371
Bartek J, Bartkova J, Taylor-Papadimitrou J, Rejthar A, Kovarik J, Lukas Z, Vojtesek B (1986) Differential expression of keratin 19 in normal human epithelial tissues revealed by monospecific monoclonal antibodies. Histochem J 18:565–575
Cooper D, Schermer A, Sun T-T (1985) Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: Strategies, applications and limitations. Lab Invest 52:243–256
Cotsarelis G, Cheng S-Z, Dong G, Sun T-T, Lavker RM (1989) Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications of epithelial stem cells. Cell 57:201–209
Cotton DWK (1986) Immunohistochemical staining of normal sweat gland. Br J Dermatol 114:441–449
Franke WW, Moll R (1987) Cytoskeletal components of lymphoid organs. I. Synthesis of cytokeratin 8 and 18 and desmin in subpopulations of extrafollicular reticulum cells of human lymph nodes, tonsils and spleen. Differentiation 36:145–163
Franke WW, Appelhans B, Schmid E, Freudenstein C, Osborn M, Weber K (1979) Identification and characterization of epithelial cells in mammalian tissues by immunofluorescence microscopy using antibodies to prekeratin. Differentiation 15:7–25
Freeman RG, Knox JM, Spiller WF (1961) Eccrime poroma. Am J Clin Pathol 5:444–450
Fuchs E, Green H (1980) Changes in keratin gene expression during terminal differentiation of the keratinocytes. Cell 19:1033–1042
Glenney J (1986) Antibody probing of Western blots which have been stained with india ink. Anal Biochem 156:315–319
Goldner R (1970) Eccrine poromatosis. Arch Dermatol 101:606–608
Gschnait F, Horn F, Lindlbauer R, Sponer D (1980) Eccrine porocarcinoma. J Cutan Pathol 7:349–353
Hashimoto K, Lever WF (1964) Eccrine poroma. J Invest Dermatol 46:237–247
Heid HW, Werner E, Franke WW (1986) The complement of α-keratin polypeptides of hair-forming cells: A subset of eight polypeptides that differ from epithelial cytokeratins. Differentiation 32:101–119
Heid HW, Moll I, Franke WW (1988) Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. I. Human and bovine hair follicles. Differentiation 37:137–157
Heid HW, Moll I, Franke WW (1988) Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. II. Concomitant and mutually exclusive synthesis of trichocytic and epithelial cytokeratins in diverse human and bovine tissues (hair follicles, nail bed and nail matrix, lingual papilla, thymic reticulum). Differentiation 37:215–230
Holubar K, Wolff K (1969) Intra-epidermal eccrine poroma. Cancer 23:626–635
Hunter JAA, Mottaz JM, Zelickson AS (1970) Ultrastructural histochemical observations on UV-irradiated human melanocytes. J Invest Dermatol 54:213–215
Huszar M, Gigi-Leitner O, Moll R, Franke WW, Geiger B (1986) Monoclonal antibodies to various acidic (type I) cytokeratins of stratified epithelia. Selective markers for stratification and squamous cell carcinomas. Differentiation 31:141–153
Hyman AB, Brownstein MH (1969) Eccrine poroma. Dermatologica 138:29–38
Krinitz K (1967) Ein Beitrag zur Klinik und Histologie des ekkrinen Poroms. Hautarzt 11:504–508
Krinitz K (1972) Malignes intraepidermales ekkrines Porom. Zbl Haut 47:9–17
Lavker RM, Sun T-T (1983) Epidermal stem cells. J Invest Dermatol 81:121s-127s
Lobitz WC Jr, Holyoke JB, Montagna W (1954) The epidermal eccrine sweat duct unit: A morphologic and biologic entity. J Invest Dermatol 22:157–158
Lynch MH, O'Guin WM, Hardy C, Mak L, Sun T-T (1986) Acidic and basic hair/nail (‘hard’) keratins: their colocalization in upper cortical and cuticle cells of human hair follicle and their relationship to ‘soft’ keratins. J Cell Biol 103:2593–2606
Makin CA, Bobrow LG, Bodmer WF (1984) Monoclonal antibody to cytokeratin for use in routine histopathology. J Clin Pathol 37:975–983
Mehregan AH, Hashimoto K, Rahbari H (1983) Eccrine adenocarcinoma. Arch Dermatol 119:104–114
Mishima Y, Morioka S (1969) Oncogenic differentiation of the intraepidermal eccrine sweat duct: eccrine poroma, poroepithelioma and porocarcinoma. Dermatologica 138:238–250
Moll R (1987) Epithelial tumor markers: cytokeratins and tissue polypeptide antigen (TPA). Curr Top Pathol 77:71–101
Moll R, Franke WW, Schiller DL, Geiger B, Krepler R (1982) The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell 31:11–24
Moll R, Franke WW, Volc-Platzer B, Krepler R (1982) Different keratin polypeptides in epidermis and other epithelia of human skin: A specific cytokeratin of molecular weight 46,000 in epithelia of the pilosebaceous tract and basal cell epitheliomas. J Cell Biol 95:285–295
Moll R, Krepler R, Franke WW (1983) Complex cytokeratin polypeptide patterns observed in certain human carcinomas. Differentiation 23:256–269
Moll R, Cowin P, Kapprell H-P, Franke WW (1986) Desmosomal proteins: new markers for identification and classification of tumors. Lab Invest 54:4–25
Moll I, Heid H, Franke WW, Moll R (1987) Distribution of a special subset of keratinocytes characterized by the expression of cytokeratin 9 in adult and fetal human epidermis of various body sites. Differentiation 33:254–265
Moll R, AchtstÄtter T, Becht E, Balcarova-StÄnder J, Ittensohn M, Franke WW (1988) Cytokeratins in normal and malignant transitional epithelium: Maintenance of expression of urothelial differentiation features in transitional cell carcinomas and bladder carcinoma cell culture lines. Am J Pathol 132:123–144
Moll I, Heid H, Franke WW, Moll R (1988) Patterns of expression of trichocytic and epithelial cytokeratins in diverse mammalian tissues. III. Hair and nail formation during human fetal development. Differentiation 139:167–184
Moll I, Heid HW, Moll R (1988) Cytokeratin analysis of pilomatrixoma: change in cytokeratin-type expression during differentiation. J Invest Dermatol 91:251–257
Moll R, Schiller DL, Franke WW (1990) Identification of protein IT of the intestinal cytoskeleton as a novel type I cytokeratin with unusual properties and expression patterns. J Cell Biol 111:567–580
Murphy GF, Bhan AK, Sato S, Mihm MC Jr, Harrist TJ (1981) A new immunologic marker for Langerhans cells. N Engl J Med 34:791–792
Murphy GF, Flynn TC, Rice RH, Pinkus GS (1984) Involucrin expression in normal and neoplastic human skin: a marker for keratinocyte differentiation. J Invest Dermatol 82:453–457
Noda Y, Kumasa S, Higashiyama H, Mori M (1988) Immuno-localization of keratin proteins in sweat gland tumours by the use of monoclonal antibody. Pathol Res Pract 183:284–291
O'Farrell PZ, Goodman HM, O'Farrell PH (1977) High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell 12:1133–1142
Pinkus H (1939) The wall of the intraepidermal part of the sweat duct. J Invest Dermatol 2:175–186
Pinkus H, Mehregan AH (1963) Epidermotropic eccrine carcinoma. Arch Dermatol 88:597–606
Pinkus H, Tanay A (1964) Die Embryologie der Haut. In: Marchionini A (Hrsg) Handbuch der Haut- und Geschlechts-krankheiten. Springer, Berlin Heidelberg Göttingen, p 624
Pinkus H, Rogin JR, Goldman P (1956) Eccrine poroma. Arch Dermatol 74:511–521
Requena L, Sánchez M, Aguilar A, Ambrojo P, Sánchez Yus E (1990) Periungual porocarcinoma. Dermatologica 180:177–180
Romeis B (1968) Mikroskopische Technik. R. Oldenbourg, München
Ryan JF, Darley CR, Pollock DJ (1986) Malignant eccrine poroma: report of three cases. J Clin Pathol 39:1099–1104
Sanderson KV, Ryan EA (1963) The histochemistry of eccrine poroma. Br J Dermatol 75:86–88
Schaarschmidt H, Wollina U (1990) Immunohistochemischer Nachweis von Calmodulin. Dermatol Monatsschr 176:363–370
Schermer A, Calvin S, Sun T-T (1986) Differentiation-related expression of a major 64 K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol 103:49–62
Skerrow D, Skerrow CJ (1983) Tonofilament differentiation in human epidermis, isolation and polypeptide chain composition of keratinocyte subpopulations. Exp Cell Res 143:27–35
Stoler A, Kopan R, Duvi M, Fuchs E (1988) Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal epidermal differentiation. J Cell Biol 107:427–466
Tölle H-G, Weber K, Osborn M (1985) Microinjection of monoclonal antibodies specific for one intermediate filament protein in cells containing multiple keratins allow insight into the composition of particular 10 mm filament. Eur J Cell Biol 38:234–244
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA 76:4350–4354
Troyanovsky SM, Guelstein VI, Tchipysheva TA, Krutovskikh VA, Bannikov GA (1989) Patterns of expression of keratin 17 in human epithelia: dependency on cell position. J Cell Sci 93:419–426
Van Muijen GNP, Ruiter DJ, Franke WW, AchtstÄtter T, Haasnoot WHB, Ponec M, Warnaar SO (1986) Cell type heterogeneity of cytokeratin expression in complex epithelia and carcinomas demonstrated by monoclonal antibodies specific for cytokeratins nos. 4 and 13. Exp Cell Res 162:97–113
Van Muijen GNP, Ruiter DJ, Warnaar SO (1987) Coexpression of intermediate filament polypeptides in human fetal and adult tissues. Lab Invest 57:359–369
Weiss RA, Eichner R, Sun T-T (1984) Monoclonal antibody analysis of keratin expression in epidermal disease: a 48-kD and a 56-kD keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol 98:1397–1406
Wild GA, Mischke D (1986) Variation of frequency of cytokeratin polypeptide patterns in human squamous non-keratinizing epithelium. Exp Cell Res 162:114–126
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moll, I., Moll, R. Comparative cytokeratin analysis of sweat gland ducts and eccrine poromas. Arch Dermatol Res 283, 300–309 (1991). https://doi.org/10.1007/BF00376618
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00376618