Summary
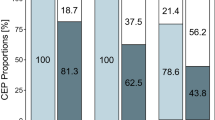
An experiment was designed to quantify the inhibitory effect of cold pressor pain (ice water immersion) on brain potentials evoked by acute laser-induced pain. One hand (including the C7 dermatome) was immersed into ice water and simultaneously the laser-evoked brain potentials were elicited consecutively from the right and left lower forearms within the C7 dermatomes. The cold pressor pain was continuously rated on a visual analogue scale (VAS). The size of the averaged brain potentials evoked from the ipsi- and contralateral C7 dermatomes were reduced significantly during cold pressor pain. The largest decrease was obtained for the potentials evoked within the C7 dermatome ipsilateral to the cold pressor pain. The size (power) of the brain potentials to individual stimuli could be measured and was correlated to the intensity of the cold pressor pain (VAS score). During the onset phase of cold pressor pain, there was a decrease in power of the individual brain potentials evoked from the C7 dermatome contralateral to the cold pressor pain but the potentials, evoked from the C7 dermatome ipsilateral to cold pressor pain, were decreased more. The overall rating of the laser-induced pain intensity showed that the perceived intensity was lowest when evoked from the dermatome concurrently exposed to cold pressor pain.
Similar content being viewed by others
References
Arendt-Nielsen L (1990) First pain related evoked potentials to argon laser-stimuli: recording and quantification. J Neurol Neurosurg Psychiat 53:398–404
Arendt-Nielsen L, Bjerring P (1988) Sensory and pain threshold characteristics to laser stimuli. J Neurol Neurosurg Psychiat 51:35–42
Arendt-Nielsen L, Zachariae R, Bjerring P (1990) Quantitative evaluation of hypnotically suggested hyperaesthesia and analgesia by painful laser stimulation. Pain 42:243–251
Arendt-Nielsen L, Svensson P, Bjerring P, Brennum J (1991) Reduced variability of pain-evoked brain potentials by new stimulation paradigms. Proceedings of 15th Annual Meeting, Scandinavian Association for the Study of Pain, p 55
Bini G, Cruccu G, Hagbarth K-E, Schady W, Torebjörk E (1984) Analgesic effect of vibration and cooling on pain induced by intraneural electrical stimulation. Pain 18:239–248
Blitz B, Dinnerstein AJ (1968) Pain attenuation by contralateral cold. Psychonomic Sci 10:395–396
Cadden SW, Villanueva L, Chitour D, Le Bars D (1983) Depression of activities of dorsal horn convergent neurones by propriospinal mechanisms triggered by noxious inputs; Comparison with diffuse noxious inhibitory controls (DNIC). Brain Res 275:1–11
Cervero F, Iggo A, Ogawa H (1976) Nociceptor-driven dorsal horn neurones in the lumbar spinal cord of the cat. Pain 2:5–24
Chen ACN, Treede R-D, Bromm B (1985) Tonic pain inhibits phasic pain: evoked cerebral potential correlates in man. Psychiatry Res 14:343–351
Ellis M (1961) The relief of pain by cooling of the skin. Br Med J 1:250–252
Fitzgerald M (1982) The contralateral input to the dorsal horn of the spinal cord in the decerebrate spinal cat. Brain Res 236:275–287
Gammon GD, Starr I (1941) Studies on the relief of pain by counterirritation. J Clin Invest 20:13–20
Handwerker HO, Iggo A, Zimmermann M (1975) Segmental and supraspinal actions on dorsal horn neurons responding to noxious and non-noxious skin stimuli. Pain 1:147–165
Hardy JD, Woolf HG, Goodell H (1940) Studies on pain. A new method for measuring pain threshold: observations on spatial summation of pain. J Clin Invest 19:649–657
Hardy JD, Woolf HG, Goodell H (1967) Pain sensations and reactions. Hatner, New York
Leandri M, Campbell JA, Lahuerta J (1985) The effect of attention on tooth-pulp evoked potentials. In: Fields HL, Dubner R, Cervero F (eds) Advances in pain research and therapy, vol 9. Raven Press, New York, pp 331–336
Le Bars D, Villanueva L (1988) Electrophysiological evidence for the activation of descending inhibitory controls by nociceptive afferent pathways. In: Fields HL, Besson JM (eds) Progress in brain research, vol 77. Elsevier, Amsterdam, pp 275–299
Le Bars D, Dickenson AH, Besson JM (1979a) Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain 6:305–327
Le Bars D, Dickenson AH, Besson JM (1979b) Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain 6:283–304
Le Bars D, Dickenson AH, Besson JM, Villanueva L (1986) Aspects of sensory processing through convergent neurons. In: Yaksh TL (ed) Spinal afferent processing. Plenum Press, New York, pp 467–504
Loeser JD, Black RG, Christman A (1975) Relief of pain by transcutaneous stimulation. J Neurosurg 42:308–314
Melzack R, Wall PD (1965) Pain mechanisms: a new theory. Science 150:971–979
Murray FS, Weaver MM (1975) Effects of ipsilateral and contralateral counterirritation on experimentally produced itch in human beings. J Comp Physiol Psychol 89:819–826
Pertovaara A, Kemppainen P, Johansson G, Karonen S-L (1982) Ischemic pain nonsegmentally produces a predominant reduction of pain and thermal sensitivity in man: a selective role for endogenous opioids. Brain Res 251:83–92
Talbot JD, Duncan GH, Bushnell MC, Boyer M (1987) Diffuse noxious inhibitory controls (DNICs): psychophysical evidence in man for intersegmental suppression of noxious heat perception by cold pressor pain. Pain 30:221–232
Wahren LK, Torebjörk E, Jörnum E (1989) Central suppression of cold-induced C fiber pain by myelinated fibre input. Pain 38:313–319
Willer JC, Roby A, Le Bars D (1984) Psychophysical and electrophysiological approaches to the pain-relieving effects of heterotopic nociceptive stimuli. Brain 107:1095–1112
Willer JC, Broucker TD, Le Bars D (1989) Encoding of nociceptive thermal stimuli by diffuse noxious inhibitory controls in humans. J Neurophysiol 5:1028–1038
Woolf S, Hardy JD (1941) Studies on pain. Observations on pain due to local cooling and on factors involved in the “cold pressor” effect. J Clin Invest 20:521–533
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arendt-Nielsen, L., Gotliebsen, K. Segmental inhibition of laser-evoked brain potentials by ipsi- and contralaterally applied cold pressor pain. Europ. J. Appl. Physiol. 64, 56–61 (1992). https://doi.org/10.1007/BF00376441
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00376441