Summary
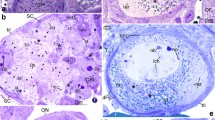
The developmental potential of the cells of the somatic follicular epithelium (follicle cells) was studied in mutants in which the differentiation of the germ-line cells is blocked at different stages of oogenesis. In two mutants, sn 36a and kelch, nurse cell regression does not occur, yet the follicle cells around the small oocyte continue their normal developmental program and produce an egg shell with micropylar cone and often deformed operculum and respiratory appendages. Neither the influx of nurse cell cytoplasm into the oocyte nor the few follicle cells covering the nurse cells are apparently required for the formation of the egg shell. In the tumor mutant benign gonial cell neoplasm (bgcn) the follicle cells can also differentiate to some extent although the germ-line cells remain morphologically undifferentiated. Vitelline membrane material was synthesized by the follicle cells in some bgcn chambers and in rare cases a columnar epithelium, which resembled morphologically that of wild-type stage-9 follicles, formed around the follicle's posterior end. The normal polarity of the follicular epithelium that is characteristic for mid-vitellogenic stages may, therefore, be established in the absence of morphologically differentiating germ-line cells. However, the tumorous germ-line cells do not constitute a homogeneous cell population since in about 30% of the analyzed follicles a cell cluster at or near the posterior pole can be identified by virtue of its high number of concanavalin A binding sites. This molecular marker reveals an anteroposterior polarity of the tumorous chambers. In follicles mutant for both bgcn and the polarity gene dicephalic the cluster of concanavalin A-stained germ-line cells shifts to more anterior positions in the follicle.
Similar content being viewed by others
References
Bender HA (1960) Studies on the expression of various singed alleles in Drosophila melanogaster. Genetics 45:867–883
Bohrmann J, Sander K (1987) Aberrant oogenesis in the patterning mutant dicephalic of Drosophila melanogaster: time-lapse recordings and volumetry in vitro. Wilhelm Roux's Arch 196:279–285
Brower DL, Smith M, Wilcox M (1981) Differentiation within the gonad of Drosophila melanogaster revealed by immunofluorescence. J Embryol Exp Morphol 63:233–242
Capco DG, McGaughey RW (1986) Cytoskeletal reorganisation during early mammalian development. Analysis using embedment-free sections. Dev Biol 115:446–458
Capco DG, Krochmalnic G, Penman S (1984) A new method of preparing embedment-free sections for transmission electron microscopy: Applications to the cytoskeletal framework and other three dimensional networks. J Cell Biol 98:1878–1885
Fasano L, Kerridge S (1988) Monitoring positional information during oogenesis in adult Drosophila. Development 104:245–253
Frey A (1986) Ursachen und Auswirkungen der veränderten Follikel-Polarität bei der Drosophila-Mutante dicephalic: experimentelle und morphologische Befunde. PhD thesis, Freiburg
Frey A, Gutzeit H (1986) Follicle cells and germ line cells both affect polarity in dicephalic chimeric follicles of Drosophila. Wilhelm Roux's Arch 195:527–532
Frey A, Sander K, Gutzeit H (1984) The spatial arrangement of germ line cells in ovarian follicles of the mutant dicephalic in Drosophila melanogaster. Wilhelm Roux's Arch 193:388–393
Gateff E (1982) Gonial cell neoplasm of genetic origin affecting both sexes of Drosophila melanogaster. In: Burger MM, Weber R (eds) Embryonic development, part B. Cellular aspects. Liss, New York, pp 621–632
Giorgi F, Postlethwait JH (1985) Development of gap junctions in normal and mutant ovaries of Drosophila melanogaster. J Morphol 185:115–129
Gutzeit HO (1986) The role of microfilaments in cytoplasmic streaming in Drosophila follicles. J Cell Sci 80:159–169
Gutzeit HO, Heinrich U-R (1981) Abnormal organization of ovarian follicles in the mutant l(1)su(f) mad-ts of Drosophila melanogaster. Experientia 37:1192–1193
Gutzeit HO, Koppa R (1982) Time-lapse film analysis of cytoplasmic streaming during late oogenesis of Drosophila. J Embryol Exp Morphol 67:101–111
Jürgens G (1977) Musterbildung in den Imaginalscheiben von Drosophila melanogaster: Entwicklungsgenetische Untersuchungen zur Entstehung und Entwicklung duplizierter Beine in einer neuen temperatursensitiven Mutante. PhD thesis, University of Freiburg i. Br.
King RC (1970) Ovarian development in Drosophila melanogaster. Academic Press, New York
King RC, Bahns M, Horowitz R, Larramendi P (1978) A mutation that affects female and male germ cells differently in Drosophila melanogaster Meigen (Diptera: Drosophilidae). Int J Insect Morphol Embryol 7:359–375
Liu X, Lorenz L, Yu Q, Hall JC, Rosbash M (1988) Spatial and temporal expression of the period gene in Drosophila melanogaster. Genes Dev 2:228–238
Lohs-Schardin M (1982) Dicephalic — a Drosophila mutant affecting polarity in follicle organization and embryonic pattern. Wilhelm Roux's Arch 191:28–36
Lohs-Schardin M, Sander K (1976) A dicephalic monster embryo of Drosophila melanogaster. Wilhelm Roux's Arch 179:159–162
Margaritis LH (1985) Structure and physiology of the egg shell. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology, vol. 1. Pergamon Press, Oxford, pp 153–230
Parks S, Spradling A (1987) Spatially regulated expression of chorion genes during Drosophila oogenesis. Genes Dev 1:497–509
Robb JA (1969) Maintenance of imaginal discs of Drosophila melanogaster in chemically defined media. J Cell Biol 186:351–362
Schüpbach T (1987) Germ line cells and soma cooperate during oogenesis to establish the dorsoventral pattern of egg shell and embryo in Drosophila melanogaster. Cell 49:699–707
Steward R, Nüsslein-Volhard C (1986) The genetics of the dorsal bicaudal D region of Drosophila melanogaster. Genetics 113:665–678
Storto PD, King RC (1988) Multiplicity of functions for the otu gene products during Drosophila oogenesis. Dev Genet 9:91–120
Strauß A (1988) Zelldifferenzierung in den Ovariolen der Drosophila Mutante benign gonial cell neoplasm. Diplomarbeit, University of Freiburg i. Br.
Warn RM, Gutzeit HO, Smith L, Warn A (1985) F-actin rings are associated with the ring canals of the Drosophila egg chamber. Exp Cell Res 157:355–363
Went DF, Junquera P (1981) Embryonic development of insect eggs formed without follicular epithelium. Dev Biol 86:100–110
Wilson TG (1980) Studies on the female-sterile phenotype of l(1), su(f) ts76a, a temperature-sensitive allele of the suppressor of forked mutation in Drosophila melanogaster. J Embryol Exp Morphol 55:247–256
Zissler D, Sander K (1973) The cytoplasmic architecture of the egg cell of Smittia spec. (Diptera, Chironomidae) I. Anterior and posterior pole regions. Wilhelm Roux's Arch 172:175–186
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gutzeit, H.O., Strauß, A. Follicle cell development is partly independent of germ-line cell differentiation in Drosophila oogenesis. Roux's Arch Dev Biol 198, 185–190 (1989). https://doi.org/10.1007/BF00375904
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00375904