Abstract
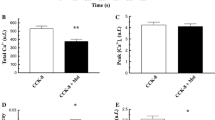
In order to establish a regulatory role for phosphoproteins in the process of receptor-stimulated Ca2+ mobilization, isolated pancreatic acinar cells, loaded with fura-2, were stimulated with cholecystokin-in-octapeptide (CCK8) in the presence of either staurosporine, a general inhibitor of protein kinase activity, or 12-O-tetradecanoylphorbol 13-acetate (TPA), an activator of protein kinase C. Staurosporine alone did not affect the average free cytosolic Ca2+ concentration ([Ca2+]i,av) in a suspension of acinar cells. However, in the presence of 1.0 μM Staurosporine the stimulatory effect of submaximal concentrations of CCK8 was significantly enhanced. The potentiating effect of the inhibitor was paralleled by the increased production of inositol 1,4,5-trisphosphate. In addition, staurosporine evoked a transient increase in [Ca2+]i,av in cells prestimulated with a submaximal concentration of CCK8. The data obtained with staurosporine indicate that CCK8-stimulated phosphorylations exert a negative feedback role in the process of receptor-mediated Ca2+ mobilization. The involvement of protein kinase C was investigated by studying the effects of TPA on CCK8-induced Ca2+ mobilization. The phorbol ester induced a rightward shift of the dose/response curve for the CCK8-evoked increase in [Ca2+]i,av, which, in contrast to the unlimited shift obtained with the receptor antagonist D-lorglumide, reached a maximum of approximately one order of a magnitude at 10 nM TPA. The inhibitory effect of TPA was completely overcome by CCK8 at concentrations at or beyond 10 nM. This observation has led to the hypothesis that protein kinase C, directly or indirectly, converts the CCK receptor from a high-affinity state to a low-affinity state. Substantial evidence in favour of this hypothesis was provided by the observation that the increase in [Ca2+]i,av evoked by the CCK8 analogue JMV-180, which acts as an agonist at the high-affinity receptor, was completely blocked by TPA pretreatment. TPA also evoked a rightward shift of the dose/response curve for the carbachol-induced increase in [Ca2+]i,av, indicating that the protein-kinase-C-mediated transition of the affinity state of receptors is a more general phenomenon. In the presence of submaximal CCK8 concentrations, TPA dose-dependently decreased the poststimulatory elevated [Ca2+]i,av to the prestimulatory level, indicating that protein kinase C also inhibits the process of sustained Ca2+ mobilization. The effects of TPA were counteracted by staurosporine, suggesting that the effects of the inhibitor itself were indeed due to inhibition of the receptor-mediated activation of protein kinase C. The data presented are in support of a negative-feedback role for protein kinase C in the process of receptor-mediated Ca2+ mobilization by a process that involves phosphorylation of the CCK receptor, thereby transforming it from a high-affinity state into a low-affinity state.
Similar content being viewed by others
References
Ansah T-A, Dho S, Case RM (1986) Calcium concentration and amylase secretion in guinea pig pancreatic acini: interactions between carbachol, cholecystokinin octapeptide and the phorbol ester, 12-O-tetradecanoylphorbol 13-acetate. Biochim Biophys Acta 889:326–333
Arita Y, Kimura T, Ogami Y, Nawata H (1991) Effects of H7 and staurosporine on cytosolic free calcium and amylase secretion in rat pancreatic acini. Pancreas 6:112–119
Berridge MJ (1987) Inositol trisphosphate and diacylglycerol: two interacting messengers. Annu Rev Biochem 56:159–193
De Pont JJHHM, Fleuren-Jakobs AMM (1984) Synergistic effect of A23187 and a phorbol ester on amylase secretion from rabbit pancreatic acini. FEBS Lett 170:64–68
Ederveen AGH, Van Emst-De Vries SE, De Pont JJHHM, Willems PHGM (1990) Dissimilar effects of the protein kinase C inhibitors, staurosporine and H7, on cholecystokinin-induced enzyme secretion from rabbit pancreatic acini. Eur J Biochem 193:291–295
Ederveen AGH, Van Emst-De Vries SE, Burgers LHC, De Pont JJHHM (1992) Purification and characterization of rabbit pancreas protein kinase C. Pancreas 7:34–44
Habara Y, Kanno T (1991) Dose-dependency in spatial dynamics of [Ca2+]c in pancreatic acinar cells. Cell Calcium 12:533–542
Kikkawa U, Nishizuka Y (1986) The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol 2:149–178
Klueppelberg UG, Gates LK, Gorelick FS, Miller LJ (1991) Agonist-regulated phosphorylation of the pancreatic cholecystokinin receptor. J Biol Chem 266:2403–2408
Kobayashi E, Nakano H, Morimoto M, Tamaoki T (1989) Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun 159:548–553
Krims PE, Pandol SJ (1988) Free cytosolic calcium and secretagogue-stimulated initial pancreatic exocrine secretion. Pancreas 3:383–390
Lee PC, Leung YK, Srimaruta N, Cumella J, Rossi T (1987) Phorbol ester attenuates cholecystokinin-stimulated amylase release in pancreatic acini of rats. Biochim Biophys Acta 931:101–109
Loessberg PA, Zhao H, Muallem S (1991) Synchronized oscillation of Ca2+ entry and Ca2+ release in agonist-stimulated AR42J cells. J Biol Chem 266:1363–1366
Louie DS, Owyang C (1991) Carbachol acts through protein kinase C to modulate cholecystokinin receptors on pancreatic acini. Am J Physiol 261:G981-G986
Matozaki T, Williams JA (1989) Multiple sources of 1,2-di-acylglycerol in isolated rat pancreatic acini stimulated by cholecystokinin. J Biol Chem 264:14 729–14 734
Matozaki T, Martinez J, Williams JA (1989) A new CCK analogue differentiates two functionally distinct CCK receptors in rat and mouse pancreatic acini. Am J Physiol 257:G594-G600
Matozaki T, Göke B, Tsunoda Y, Rodriguez M, Martinez J, Williams JA (1990) Two functionally distinct cholecystokinin receptors show different modes of actions on Ca2+ mobilization and phospholipid hydrolysis in isolated rat pancreatic acini. J Biol Chem 265:6247–6254
Muallem S, Schoeffield MS, Fimmel CJ, Pandol SJ (1988) Agonist-sensitive calcium pool in the pancreatic acinar cell. I. Permeability properties. Am J Physiol 255:G221-G228
Nishizuka Y (1986) Studies and perspectives of protein kinase C. Science 233:305–312
Ochs DL, Korenbrot JI, Williams JA (1985) Relation between free cytosolic calicum and amylase release by pancreatic acini. Am J Physiol 249:G389-G398
Osipchuk YV, Wakui M, Yule DI, Gallacher DV, Petersen OH (1990) Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+: simultaneous microfluorimetry and Ca2+ dependent Cl− current recording in single pancreatic acinar cells. EMBO J 9:697–704
Pandol SJ, Schoeffield MS, Sachs G, Muallem S (1985) Role of cytosolic calcium in secretagogue-stimulated amylase release from dispersed acini from guinea pig pancreas. J Biol Chem 260:10 081–10 086
Pralong WF, Wollheim CB, Bruzzone R (1988) Measurement of cytosolic free Ca2+ in individual pancreatic acini. FEBS Lett 242:79–84
Roe MW, Lemasters JJ, Herman B (1990) Assessment of fura-2 for measurements of cytosolic free calcium. Cell Calcium 11:63–73
Saluja AK, Dawra RK, Lerch MM, Steer ML (1992) CCK-JMV-180, an analog of cholecystokinin, releases intracellular calcium from an inositol trisphosphate-independent pool in rat pancreatic acini. J Biol Chem 267:11 202–11 207
Sato S, Stark HA, Martinez J, Beaven MA, Jensen RT, Gardner JD (1989) Receptor occupation, calcium mobilization, and amylase release in pancreatic acini: effect of CCK-JMV-180. Am J Physiol 257:G202-G209
Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F (1986) Staurosporine, a potent inhibitor of phospholipid/Ca2+ dependent protein kinase. Biochem Biophys Res Comun 135:397–402
Tepikin AV, Voronina SG, Gallacher DV, Petersen OH (1992) Acetylcholine-evoked increase in the cytoplasmic Ca2+ concentration and Ca2+ extrusion measured simultaneously in single mouse pancreatic acinar cells. J Biol Chem 267:3569–3572
Tepikin AV, Voronina SG, Gallacher DV, Petersen OH (1992) Pulsatile Ca2+ extrusion from single pancreatic acinar cells during receptor-activated cytosolic Ca2+ spiking. J Biol Chem 267:14 073–14 076
Tsunoda Y, Stuenkel EL, Williams JA (1990) Oscillatory mode of calcium signaling in rat pancreatic acinar cells. Am J Physiol 258:C147-C155
Van Haastert PJM (1989) Determination of inositol 1,4,5-trisphosphate levels in Dictyostelium by isotope dilution assay. Anal Biochem 177:115–119
Wank SA, Harkins R, Jensen RT, Shapira H, De Weerth A, Slattery T (1992) Purification, molecular cloning, and functional expression of the cholecystokinin receptor from rat pancreas. Proc Natl Acad Sci USA 89:3125–3129
Willems PHGM, Van Nooij IGP, Haenen HEMG, De Pont JJHHM (1987) Phorbol ester inhibits cholecystokinin octapeptide-induced amylase secretion and calcium mobilization, but is without effect on secretagogue-induced hydrolysis of phosphatidylinositol 4,5-bisphosphate in rabbit pancreatic acini. Biochim Biophys Acta 930:230–236
Willems PHGM, Van Den Broek BAM, Van Os CH, De Pont JJHHM (1989) Inhibition of inositol 1,4,5-trisphosphate-induced Ca2+ release in permeabilized pancreatic acinar cells by hormonal and phorbol ester pretreatment. J Biol Chem 264:9762–9767
Willems PHGM, De Jong MD, De Pont JJHHM, Van Os CH (1990) Ca2+-sensitivity of inositol 1,4,5-trisphosphate-mediated Ca2+ release in permeabilized pancreatic acinar cells. Biochem J 265:681–687
Willems PHGM, Van Emst-De Vries SE, Van Os CH, De Pont JJHHM (1993) Dose-dependent recruitment of pancreatic acinar cells during receptor-mediated calcium mobilization. Cell Calcium 14:145–159
Yule DI, Gallacher DV (1988) Oscillations of cytosolic calcium in single pancreatic acinar cells stimulated by acetylcholine. FEBS Lett 239:358–362
Yule DI, Lawrie AM, Gallacher DV (1991) Acetylcholine and cholecystokinin induce different patterns of oscillating calcium signals in pancreatic acinar cells. Cell Calcium 12:145–151
Zhang B-X, Muallem S (1992) Feedback inhibition of Ca2+ release by Ca2+ is the underlying mechanism of agonist-evoked intracellular Ca2+ oscillations in pancreatic acinar cells. J Biol Chem 267:24 387–24 393
Zhao H, Loessberg PA, Sachs G, Muallem S (1990) Regulation of intracellular Ca2+ oscillation in Ar42J cells. J Biol Chem 265:20 856–20 862
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Willems, P.H.G.M., Van Hoof, H.J.M., Van Mackelenbergh, M.G.H. et al. Receptor-evoked Ca2+ mobilization in pancreatic acinar cells: Evidence for a regulatory role of protein kinase C by a mechanism involving the transition of high-affinity receptors to a low-affinity state. Pflugers Arch. 424, 171–182 (1993). https://doi.org/10.1007/BF00374609
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00374609