Abstract
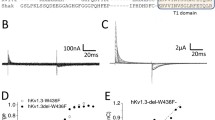
Macroscopic currents of wild-type rat brain IIA (RBIIA) and mutant Na channels were recorded in excised patches from Xenopus oocytes. A charge deletion (K859Q) and an adjacent conservative mutation (L860F) in the second domain S4 membrane-spanning region differentially altered voltage sensitivity and kinetics. Analysis of voltage dependence was confined to Na currents with fast inactivation kinetics, although RBIIA and K859Q (but not L860F) also showed proportional shifts between at least two gating modes, rendering currents with fast or slow inactivation kinetics, respectively. Compared to RBIIA, the midpoint of the activation curve was shifted in both K859Q and L860F by 22 mV to more positive potentials, yet this shift was not associated with a corresponding change in the voltage dependence of time constants for activation (τ a) or inactivation (τ h1, τ h2). L860F showed faster activation time constants τ a than RBIIA, while K859Q was slower for both the activation (τ a) and the inactivation components (τ h1). Similarly, the steady-state inactivation curve of L860F but not K859Q shifted by 9 mV in the hyperpolarizing direction. Thus, the fourth charge in the IIS4 transmembrane segment exerts control over voltage sensitivity and kinetics of activation and may interact with structure that influence other aspects of channel gating.
Similar content being viewed by others
References
Adelman WJ, Palti Y (1969) The effects of external potassium and long duration voltage conditioning on the amplitude of sodium currents in the giant axon of the squid, Loligo peali. J Gen Physiol 54:589–606
Aldrich RW, Corey CP, Stevens CF (1983) A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature 306:436–441
Armstrong CM, Bezanilla F (1977) Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol 70:567–590
Auld VJ, Goldin AL, Krafte DS, Marshall J, Dunn JM, Catterall WA, Lester HA, Davidson N, Dunn RJ (1988) A rat brain Na+ channel α subunit with novel gating properties. Neuron 1:449–461
Auld VJ, Goldin AL, Krafte DS, Catterall WA, Lester HA, Davidson N (1990) A neutral amino acid change in segment IIS4 dramatically alters the gating properties of the voltage-dependent sodium channel. Proc Natl Acad Sci USA 87:323–327
Fleig A, Ruben PC, Rayner MD (1994) Kinetic mode switch of rat brain IIA sodium channels in Xenopus oocytes excised macro patches. Pflügers Arch 427:399–405
Heggeness ST, Starkus JG (1986) Saxitoxin and tetrotoxin. Electrostatic effects on sodium channel gating in crayfish giant axons. Biophys J 49:629–643
Heinemann S, Terlau H, Stühmer W, Imoto K, Numa S (1992) Calcium channel characteristics conferred on the sodium channel by single mutations. Nature 356:441–443
Joho RH, Moorman JR, VanDongen AMJ, Kirsch GE, Silberberg H, Schuster G, Brown AM (1990) Toxin and kinetic profile of rat brain type III sodium channels expressed in Xenopus oocytes. Mol Brain Res 7:105–113
Kayano T, Noda M, Flockerzi V, Takahashi H, Numa S (1988) Primary structure of rat brain sodium channel III deduced from the cDNA sequence. FEBS Lett 228:187–194
Kunkel TA (1985) Rapid and efficient mutagenesis without phenotypic selection. Proc Natl Acad Sci USA 82:488–492
Moorman JR, Kirsch GE, VanDongen AMJ, Joho RH, Brown AM (1990) Fast and slow gating of sodium channels encoded by a single mRNA. Neuron 4:243–252
Narahashi T (1974) Chemicals as tools in the study of excitable membrane. Physiol Rev 54:813–889
Noda M, Shimizu S, Tanabe T, Takai T, Kayano T, Ikeda T, Takahashi H, Nakayama H, Kanaoka Y, Minamino N, Kangawa K, Matsuo H, Raftery M, Hirose S, Inayama H, Hayashida T, Miyata T, Numa S (1984) Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature 312:121–127
Quandt FN (1987) Burst kinetics of sodium channels which lack fast inactivation in mouse neuroblastoma cells. J Gen Physiol 392:563–585
Ruben PC, Starkus JG, Rayner MD (1992) Stready state availability of sodium channels: interactions between slow inactivation and activation. Biophys J 61:941–955
Rudy B (1978) Slow inactivation of the sodium conductance in squid giant axons. Pronase resistance. J Physiol (Lond) 283:1–21
Ruff R, Simoncini L, Stühmer W (1987) Comparison between slow sodium channel inactivation in rat slow- and fast-twitch muscle. J Physiol (Lond) 383:339–348
Starkus JG, Rayner MD, Fleig A, Ruben PC (1993) Photodynamic modification of sodium channels by methylene blue: effects on fast and slow inactivation. Biophys J 65:715–726
Stühmer W, Conti F, Suzuki H, Wang X, Noda M, Yahagi N, Kubo H, Numa S (1989) Structural parts involved in activation and inactivation of the sodium channel. Nature 339:597–603
Tytgat J, Nakazawa K, Hess P (1993) Cooperative and non-cooperative subunit interactions determine voltage-dependent K+ channel gating. Biophys J 64:A226
Vassilev PM, Scheuer T, Catterall WA (1989) Identification of an intracellular peptide segment in sodium channel inactivation. Science 241:1658–1661
West JW, Scheuer T, Maechler L, Catterall WA (1992) Efficient expression of rat brain type IIA Na+ channel α subunits in a somatic cell line. Neuron 8:59–70
Zhou J, Potts JF, Trimmer JS, Agnew WS, Sigworth FJ (1991) Multiple gating modes and the effect of modulating factors on the μI sodium channel. Neuron 7:775–785
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fleig, A., Fitch, J.M., Goldin, A.L. et al. Point mutations in IIS4 alter activation and inactivation of rat brain IIA Na channels in Xenopus oocyte macropatches. Pflügers Arch. 427, 406–413 (1994). https://doi.org/10.1007/BF00374254
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00374254