Abstract
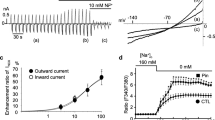
Adenosine triphosphate (ATP) dependent potassium channels (KATP channels) in heart ventricular muscle cells can be activated by depletion of intracellular ATP stores as well as by channel openers. In the present study we examined whether properties of KATP channels are dependent on the mode of activation. Whole-cell and single-channel currents were investigated by use of the patch-clamp technique in isolated ventricular rat myocytes. The channel opener rilmakalim dose dependency activated whole-cell currents [concentration for half-maximal activation (EC50) = 1.1 μM, Hill coefficient = 3.1, saturation concentration 10 μM]. Metabolic inhibition with 2-deoxy-d-glucose (10 mmol/l) also activated KATP currents after a time lag of several minutes. These currents were about two-fold higher than the rilmakalim-activated currents (rilmakalim-activated current 3.9 ±0.2nA, 2-deoxy-d-glucose-activated current 8.1±0.9 nA; both recorded at 0 mV clamp potential). While the rilmakalim-activated current could be blocked completely and with high affinity by the sulphonylurea glibenclamide [concentration for half-maximal inhibition (IC50) = 8 nM, Hill coefficient = 0.7] the 2-deoxy-d-glucose-activated current could only be blocked partially (by maximally 46%) and higher glibenclamide concentrations were needed (IC50 = 480 nM, Hill coefficient = 0.8). The partial loss of blocking efficiency after metabolic inhibition was not restricted to glibenclamide but was also observed with the sulfonylureas glimepiride and HB 985, as well as with the non-sulfonylureas HOE 511 and 5-hydroxydecanoate. Single-channel studies were in accordance with these whole-cell experiments. Both rilmakalim and metabolic inhibition with the uncoupler carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) activated single channels in the attached mode, where the number of current levels was significantly higher in the case of FCCP. Rilmakalim-activated channels were completely blocked by 10 μM glibenclamide, whereas several single-channel levels appeared in the presence of 100 μM glibenclamide after metabolic inhibition. In conclusion, after metabolic inhibition the amplitude of the activated KATP current is about twice as high as under saturating concentrations of the opener rilmakalim. Moreover, channels activated by metabolic inhibition lost part of their sensitivity to known channel blockers.
Similar content being viewed by others
References
Anderson SE, Murphy E, Steenbergen C, London RE, Cala PM (1990) Na-H exchange in myocardium: effects of hypoxia and acidification on Na and Ca. Am J Physiol 259:C940-C948
Ashcroft SJH, Ashcroft FM (1990) Properties and function of ATP-sensitive K+ channels. Cell Signal 2:197–214
Bekheit SS, Restivo M, Boutjdir M, Henkin R, Gooyandeh K, Assadi M, Khatib S, Gough WB, El-Sherif N (1990) Effects of glyburide on ischemia-induced changes in extracellular potassium and local myocardial activation: A potential new approach to management of ischemia-induced malignant ventricular arrhythmias. Am Heart J 119:1025–1033
Benndorf K, Bollmann G, Friedrich M, Hirche H (1992) Anoxia induces time-independent K+ current through K(ATP) channels in isolated heart cells of the guinea-pig. J Physiol (Lond) 454:339–357
Benndorf K, Doepner C, Gebhardt H, Hirche H, Thierfelder S (1994) Anoxia modulates two types of K+ currents in isolated ventricular cells of mice (abstract). Pflügers Arch 426: R 61
Billmann GE, Avendano CE, Halliwill JR, Burroughs JM (1993) The effects of the ATP-dependent potassium channel antagonist, glyburide, on coronary blood flow and susceptibility to ventricular fibrillation in unanesthetized dogs. J Cardiovasc Pharmacol 21:197–204
Cacciapuoti F, Spiezia R, Bianchi U, Lama D, D'Avino M, Varricchio M (1991) Effectiveness of glibenclamide on myocardial ischemic ventricular arrhythmias in non-insulin dependent diabetes mellitus. Am J Cardiol 67:843–847
Deutsch N, Weiss JN (1993) ATP-sensitive K+ channel modification by metabolic inhibition in isolated guinea-pig ventricular myocytes. J Physiol (Lond) 465:163–179
Deutsch N, Weiss JN (1994) Effect of trypsin on cardiac ATP-sensitive K+ channels. Am J Physiol 266:H613-H622
Edwards G, Weston AH (1993). The pharmacology of ATP-sensitive potassium channels. Annu Rev Pharmacol Toxicol 33:597–637
Findlay I (1987) ATP-sensitive K+ channels in rat ventricular myocytes are blocked and inactivated by internal divalent cations. Pflügers Arch 410:313–320
Findlay I (1992) Inhibition of ATP-sensitive K+ channels in cardiac muscle by the sulfonylurea drug glibenclamide. J Pharmacol Exp Ther 261:540–545
Findlay I (1993) Sulfonylurea drugs no longer inhibit ATP-sensitive K+ channels during metabolic stress in cardiac muscle. J Pharmacol Exp Ther 266:456–467
Gasser RNA, Vaughan-Jones RD (1990) Mechanism of potassium efflux and action potential shortening during ischemia in isolated mammalian cardiac muscle. J Physiol (Lond) 431:713–741
Hamada E, Takikawa R, Ito H, Iguchi M, Terano, A, Sugimoto T, Kurachi Y (1990) Glibenclamide specifically blocks ATP-sensitive K+ channel current in atrial myocytes of guinea pig heart. Jpn J Pharmacol 54:473–477
Hamill OP Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391:85–100
Isenberg G, Vereecke J, Heyden G van der, Carmeliet E (1983) The shortening of the action potential by DNP in guineapig ventricular myocytes is mediated by an increase of a time-independent K conductance. Pflügers Arch 397: 251–259
Kantor PF, Coetzee A, Carmeliet EE, Dennis ST, Opie LH (1990) Reduction of ischemic K+ loss and arrhythmias in rat hearts. Effect of glibenclamide, a sulfonylurea. Circ Res 66:478–485
Murphy E, Steenbergen C, Levy LA, Raju B, London RE (1989) Cytosolic free magnesium levels in ischemic rat heart. J Biol Chem 264:5622–5627
Nichols CG, Lederer WJ (1991) Adenosine triphosphatesensitive potassium channels in the cardiovascular system. Am J Physiol 261:H1675-H1686
Nichols CG, Lopatin AN (1993) Trypsin and α-chymotrypsin treatment abolishes glibenclamide sensitivity of KATP channels in rat ventricular myocytes. Pflügers Arch 422:617–619
Ripoll C, Lederer WJ, Nichols CG (1993) On the mechanism of inhibition of K(ATP) channels by glibenclamide in rat ventricular myocytes. J Cardiovasc Electrophysiol 4:38–47
Takano M, Noma A (1993) The ATP-sensitive K+ channel. Progr Neurobiol 41:21–30
Tercic A, Jahangir A, Kurachi Y (1994) HOE-234, a second generation K+ channel opener, antagonizes the ATP-dependent gating of cardiac ATP-sensitive K+ channels. J Pharmacol Exp Ther 268:818–824
Venkatesh N, Lamp ST, Weiss JN (1991) Sulfonylureas, ATP-sensitive K+ channels, and cellular K+ loss during hypoxia, ischemia, and metabolic inhibition in mammalian ventricle. Circ Res 69:623–637
Virág L, Furukawa T, Hiraoka M (1993) Modulation of the effect of glibenclamide on KATP channels by ATP and ADP. Mol Cell Biochem 119:209–215
Wilde AAM, Janse MJ (1994) Electrophysiological effects of ATP sensitive potassium channel modulation:implications for arrhythmogenesis. Cardiovasc Res 28:16–24
Wilde AAM, Escande D, Schumacher CA, Thuringer D, Mestre M, Fiolet JWT, Janse MJ (1990). Potassium accumulation in the globally ischemic mammalian heart. A role for the ATP-sensitive potassium channel. Circ Res 67:835–843
Yazawa K, Kaibara M, Ohara M, Kameyama M (1990) An improved method for isolating cardiac myocytes useful for patch-clamp studies. Jpn J Physiol 40:157–163
Zünkler BJ, Lenzen S, Männer K, Panten U, Trube G (1988) Concentration-dependent effects of tolbutamide, meglitinide, glipizide, glibenclamide and diazoxide on ATP-regulated K+ current in pancreatic B-cells. Naunyn Schmiedbergs Arch Pharmacol 337:225–230
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krause, E., Englert, H. & Gögelein, H. Adenosine triphosphate-dependent K currents activated by metabolic inhibition in rat ventricular myocytes differ from those elicited by the channel opener rilmakalim. Pflügers Arch. 429, 625–635 (1995). https://doi.org/10.1007/BF00373983
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00373983